Abstract
1. Renin secretion of rat renal cortical slices was measured as a function of extracellular K and ouabain concentrations in the incubation medium.
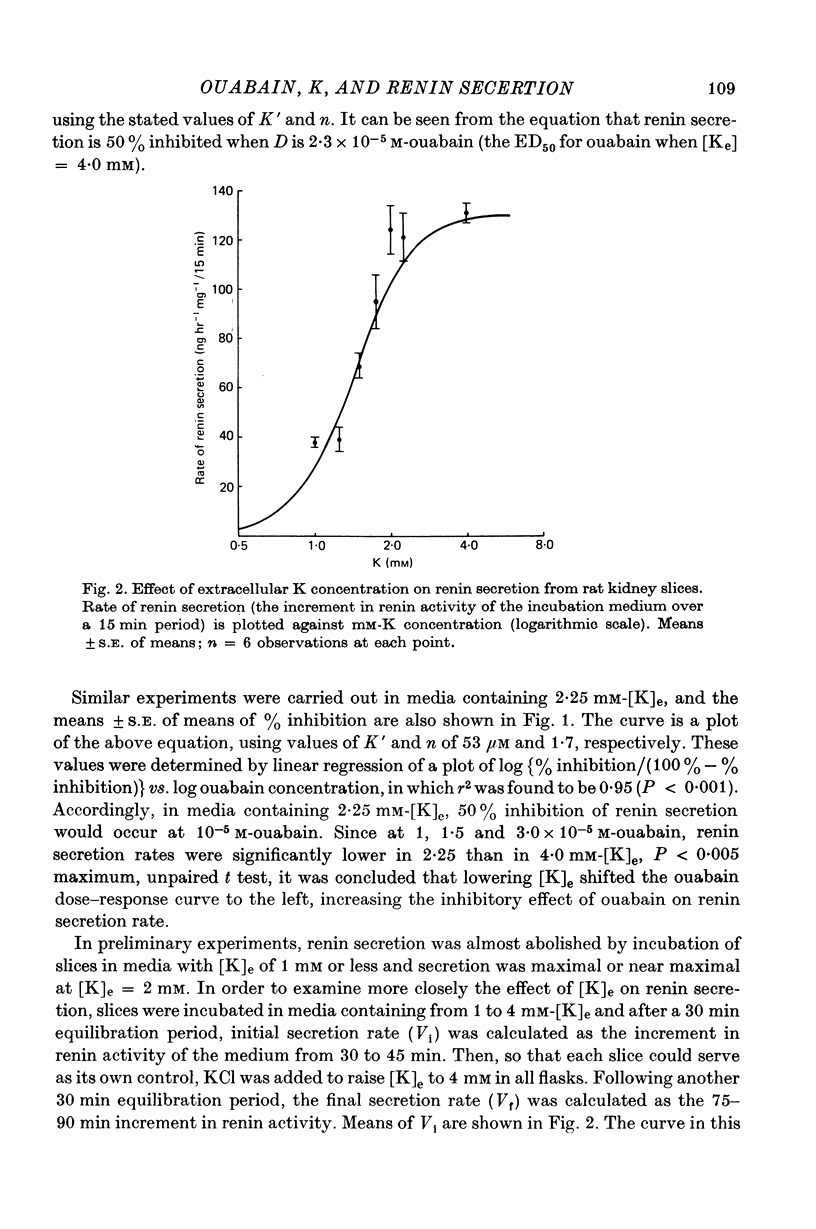
2. A sigmoid relationship was found between renin secretion and log K concentration over the range 1·0-4·0 mM. Secretion was maximal at about 2·25 mM-K and half-maximal at about 1·43 mM-K.
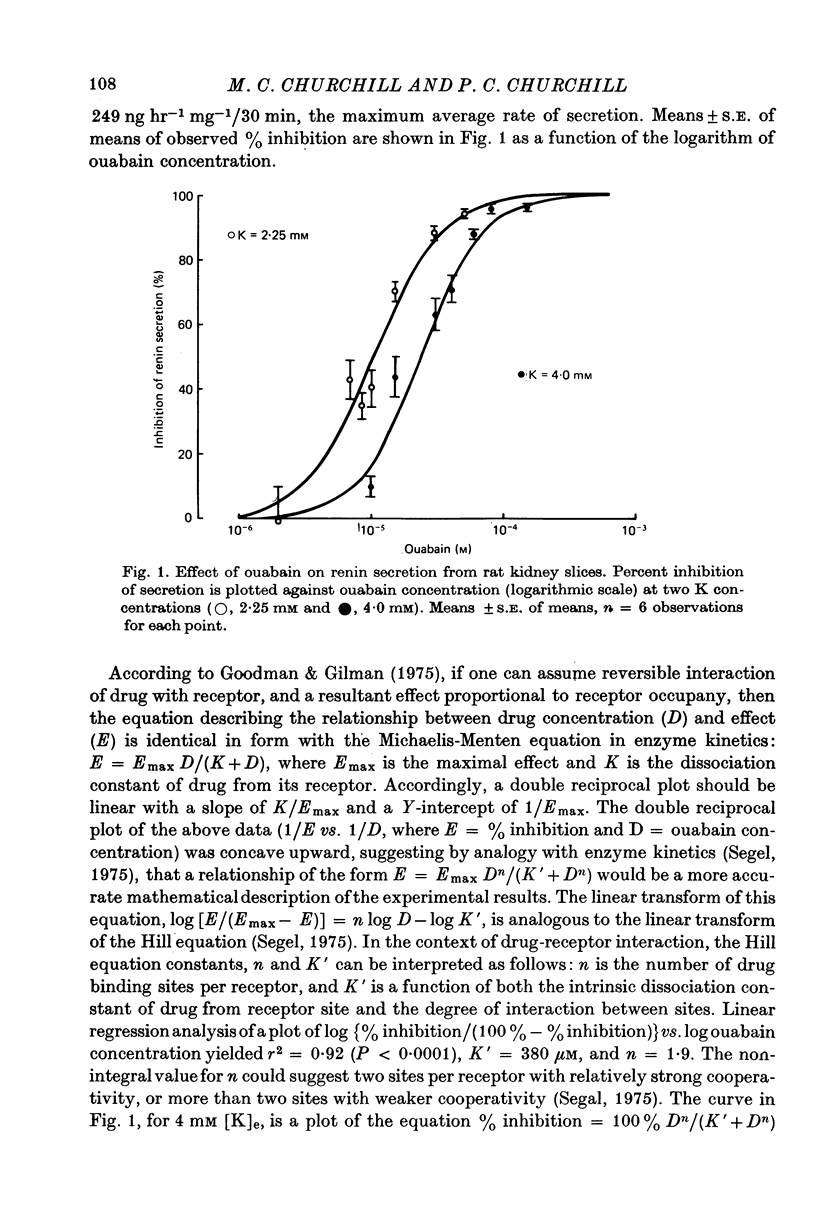
3. In media containing 4·0 mM-K, ouabain at 10-8, 10-7, and 10-6 M did not affect renin secretion. Higher concentrations of ouabain inhibited secretion. A sigmoid relationship was found between% inhibition of secretion and log ouabain concentration (10-6-10-3 M). Inhibition was half-maximal at 2·3 × 10-5 M and complete at 10-3 M-ouabain.
4. Lowering extracellular K concentration from 4·0 to 2·25 mM shifted the dose-effect curve of ouabain to the left. At 2·25 mM-K, inhibition of renin secretion was half-maximal at 10-5 M-ouabain.
5. The inhibitory effect of 2 × 10-5 M-ouabain (twice the dose for 50% inhibition) in media containing 2·25 mM-K was nearly identical to the combined effect of lowering K to 1·43 mM (the concentration required for 50% inhibition) and adding 10-5 M-ouabain. This observation suggests that ouabain and low extracellular K act at a common site, presumably on Na, K-ATPase activity, to inhibit renin secretion.
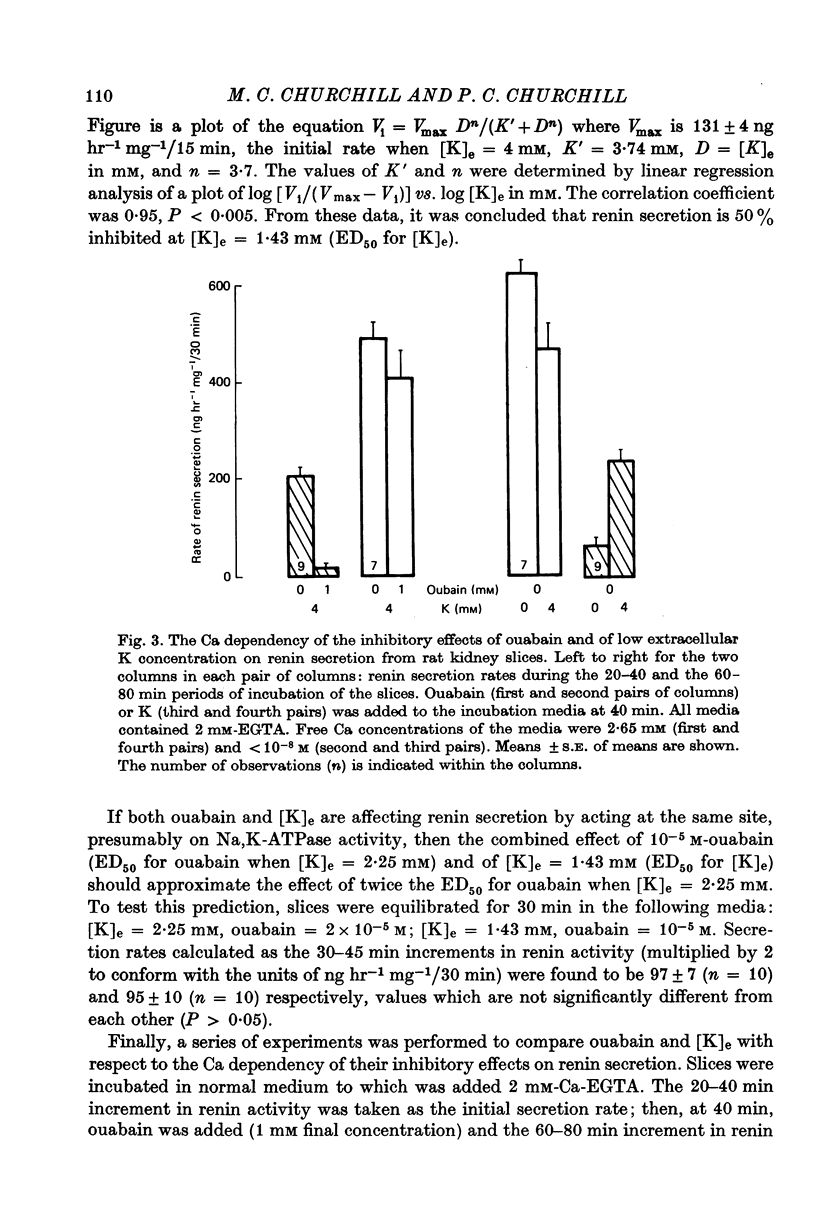
6. Neither 10-3 M-ouabain nor K-free medium inhibited renin secretion when the concentration of free Ca in the medium was lowered to < 10-8 M. Therefore it is proposed that as a result of Na, K-ATPase inhibition, (a) intracellular Na increases, (b) intracellular Ca increases via Na-Ca exchange, provided that extracellular Ca exceeds 10-8 M, and that (c) Ca accumulation, in some unknown manner, inhibits renin secretion from rat renal cortical slices.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Brody T. M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977 Sep;29(3):187–220. [PubMed] [Google Scholar]
- Barajas L. Renin secretion: an anatomical basis for tubular control. Science. 1971 Apr 30;172(3982):485–487. doi: 10.1126/science.172.3982.485. [DOI] [PubMed] [Google Scholar]
- Baumbach L., Leyssac P. P., Skinner S. L. Studies on renin release from isolated superfused glomeruli: effects of temperature, urea, ouabain and ethacrynic acid. J Physiol. 1976 Jun;258(1):243–256. doi: 10.1113/jphysiol.1976.sp011417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach L., Leyssac P. P. Studies on the mechanism of renin release from isolated superfused rat glomeruli: effects of calcium, calcium ionophore and lanthanum. J Physiol. 1977 Dec;273(3):745–764. doi: 10.1113/jphysiol.1977.sp012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine E. H., Zimmerman M. B. Renal function and renin secretion after administration of ouabain and ouabain plus furosemide in conscious sheep. Circ Res. 1978 Jul;43(1):36–43. doi: 10.1161/01.res.43.1.36. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Churchill P. C., McDonald F. D. Effect of ouabain on renin secretion in anaesthetized dogs. J Physiol. 1974 Nov;242(3):635–646. doi: 10.1113/jphysiol.1974.sp010727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C. Possible mechanism of the inhibitory effect of ouabain on renin secretion from rat renal cortical slices. J Physiol. 1979 Sep;294:123–134. doi: 10.1113/jphysiol.1979.sp012919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Fynn M., Onomakpome N., Peart W. S. The effects of ionophores (A23187 and RO2-2985) on renin secretion and renal vasoconstriction. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):199–212. doi: 10.1098/rspb.1977.0135. [DOI] [PubMed] [Google Scholar]
- Haulica I., Petrescu G., Branisteanu D., Rosca V., Balan G. Recherches sur le rôle des phénomènes membranaires dans la sécrétion de la rénine. J Physiol (Paris) 1974 Mar;68(1):17–26. [PubMed] [Google Scholar]
- Hokin L. E. Purification and properties of the (sodium + potassium)-activated adenosinetriphosphatase and reconstitution of sodium transport. Ann N Y Acad Sci. 1974;242(0):12–23. doi: 10.1111/j.1749-6632.1974.tb19075.x. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Klaus W. The subcellular basis for the mechanism of inotropic action of cardiac glycosides. Pharmacol Rev. 1971 Sep;23(3):193–261. [PubMed] [Google Scholar]
- Lyons H. J., Chruchhill P. C. Renin secretion from rat renal cortical cell suspensions. Am J Physiol. 1975 Jun;228(6):1835–1839. doi: 10.1152/ajplegacy.1975.228.6.1835. [DOI] [PubMed] [Google Scholar]
- Lyons H. J., Churchill P. C. The influence of ouabain on in vitro renin secretion and intracellular sodium. Nephron. 1975;14(6):442–450. doi: 10.1159/000180478. [DOI] [PubMed] [Google Scholar]
- Lyons H. J., Churchill P. C. The influence of ouabain on in vitro renin secretion. Proc Soc Exp Biol Med. 1974 Apr;145(4):1148–1150. doi: 10.3181/00379727-145-37970. [DOI] [PubMed] [Google Scholar]
- MCCLANE T. K. A BIPHASIC ACTION OF OUABAIN ON SODIUM TRANSPORT IN THE TOAD BLADDER. J Pharmacol Exp Ther. 1965 Apr;148:106–110. [PubMed] [Google Scholar]
- Morris B. J., Johnston C. I. Isolation of renin granules from rat kidney cortex and evidence for an inactive form of renin (prorenin) in granules and plasma. Endocrinology. 1976 Jun;98(6):1466–1474. doi: 10.1210/endo-98-6-1466. [DOI] [PubMed] [Google Scholar]
- PALMER R. F., NECHAY B. R. BIPHASIC RENAL EFFECTS OF OUABAIN IN THE CHICKEN: CORRELATION WITH A MICROSOMAL NA+-K+ STIMULATED ATP-ASE. J Pharmacol Exp Ther. 1964 Oct;146:92–98. [PubMed] [Google Scholar]
- Park C. S., Malvin R. L. Calcium in the control of renin release. Am J Physiol. 1978 Jul;235(1):F22–F25. doi: 10.1152/ajprenal.1978.235.1.F22. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Cation interactions with different functional states of the Na+, K+-ATPase. Ann N Y Acad Sci. 1974;242(0):185–202. doi: 10.1111/j.1749-6632.1974.tb19090.x. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Van Dongen R., Peart W. S. Calcium dependence of the inhibitory effect of angiotensin on renin secretion in the isolated perfused kidney of the rat. Br J Pharmacol. 1974 Jan;50(1):125–129. doi: 10.1111/j.1476-5381.1974.tb09599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander A. J. Control of renin release. Physiol Rev. 1967 Jul;47(3):359–382. doi: 10.1152/physrev.1967.47.3.359. [DOI] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. Vectorial aspects of adenosine-triphosphatase activity in erythrocyte membranes. Biochem J. 1964 Nov;93(2):337–348. doi: 10.1042/bj0930337. [DOI] [PMC free article] [PubMed] [Google Scholar]


