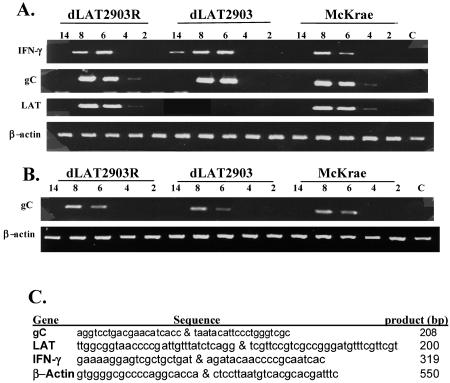
FIG. 4.
Gene expression in male mice infected with HSV-1. As described in the legend for Fig. 2, adult male (A) and female (B) BALB/c mice were infected with the designated strains of HSV-1. Brains from individual mice were collected following euthanasia and snap-frozen on dry ice. One ml of TRIZOL reagent was added (Invitrogen, Carlsbad, CA), and RNA was prepared according to the manufacturer's instructions. For the designated times after infection (numbers of days shown at the tops of the gels), RNA was prepared from brains of three mice. RNA samples were subjected to DNase I digestion to remove trace amounts of contaminating DNA. Samples from mock-infected mice are designated C. Semiquantitative reverse transcriptase PCR was performed as described previously (34) using RNA from the brain of a single mouse. RNA concentrations were determined initially by measuring the optical density at 260 nm. Running a 1.2% formaldehyde agarose gel using 0.5 to 1 μg of RNA monitored the integrity of total RNA and confirmed the optical density reading. First-strand cDNA synthesis was performed with the SuperScript first-strand synthesis system for reverse transcriptase PCR (Invitrogen) using equal amounts of total RNA (1 to 2 μg) and oligo(dT)12-16 (for cellular genes) or random hexamers (for HSV-1 genes). PCRs were carried out in 20-μl reaction mixtures that contained 0.5 μM of the designated gene-specific primers shown in panel A, 1/10 of the cDNA reaction mixture, 2.5 to 5 mM MgCl2, 25 to 100 μM dNTP, and 1 U Taq DNA polymerase. LAT cDNA was amplified using the GC-rich PCR system (Roche). Without reverse transcriptase in the reaction mixture, there were no specific amplified products (data not shown). These results are representative of three separate experiments using RNA prepared from different mice. Upper gel in panel B, IFN-γ; lower gel in panel B, β-actin. (C) All primers were described previously (32, 34) and are presented in a 5′-to-3′ direction. For each gene, the first primer is the forward primer and the second is the reverse primer.