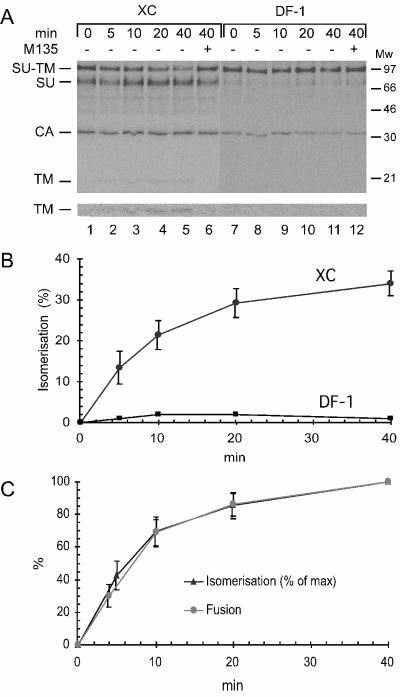
FIG. 1.
(A) SU-TM disulfide bond isomerization in XC cell-bound Mo-MLV. [35S]Cys-labeled Mo-MLV in culture medium was bound to XC or DF-1 cells for 1 h on ice and incubated in fusion buffer for 0 to 40 min at 37°C. The cultures were lysed in the presence of 20 mM NEM, and viral proteins were captured by immunoprecipitation with polyclonal antibody HE863 for nonreducing SDS-PAGE. The isomerization of the SU-TM disulfide bond was followed by a decrease in SU-TM complexes, increase in free SU, and the appearance of TM. This was observed in virus bound to XC (lanes 1 to 5) but not DF-1 (lanes 7 to 11) cells. Control samples in lanes 6 (XC cell-bound virus) and 12 (DF-1 cell-bound virus) were incubated for 40 min with 1.2 mM M135. Note that free SU does not bind to the DF-1 cells, which are receptor negative. This is in contrast to virus particles, which bind nonspecifically. The figure represents a phosphorimage of the gel. The lower part shows the bottom part of the gel with the TM band at higher contrast. (B) Quantification of isomerization. The degree of isomerization was calculated based on the incubation-induced release of SU from the SU-TM complexes and expressed as a percentage of complete isomerization. In the case of virus bound to DF-1 cells, isomerization was quantified by relating the amount of SU-TM complexes to that of the nonincubated control. (C) Correlation of kinetics of isomerization with that of fusion. The isomerization kinetics was modified from panel B by setting maximal isomerization to 100%. The fusion kinetics was determined by incubating XC cell-bound Mo-MLV at 37°C in fusion buffer for 0 to 40 min and then inactivating the virus by treatment with pH 3 buffer. The cultures were further incubated for 3 h. During this time, virus-fused cells developed into polykaryons, which were used to assess relative fusion efficiencies. Maximal fusion was achieved by 40 min of incubation, which was set to 100%. Standard deviations are indicated.