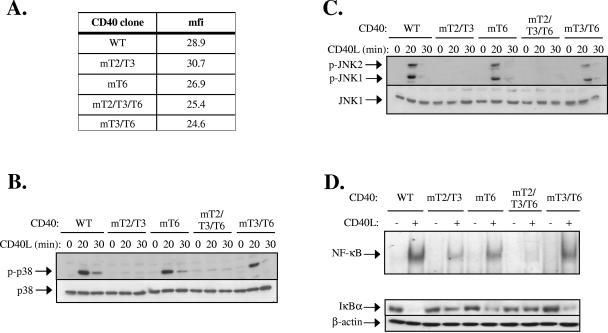
FIG. 2.
The binding of TRAF2 to the cytoplasmic tail of CD40 plays a major role in CD40 signal transduction in nonhemopoietic cells. (A) Representative flow cytometric assay showing the CD40 levels expressed in HeLa cell clones stably transfected with wild type and mutated CD40 sequences. mfi, mean fluorescence intensity. (B and C) Activation of p38 (B) and JNK (C) following CD40 activation in WT and mutated CD40-transfected HeLa cells. Cells were stimulated with 0.1 μg/ml recombinant soluble CD40L or left untreated, as indicated. Lysates were immunoblotted with antibodies specific for the phosphorylated or total p38 and JNK. At least three independent experiments were performed and gave similar results. In addition to results from the GST pull-down assays (Fig. 1B), the inability of CD40mT3/T6 to interact with TRAF3 was confirmed by immunoprecipitation assays in lysates from CD40L-stimulated HeLa-CD40mT3/T6 cells (data not shown). (D) Induction of NF-κB binding activity following CD40 activation in WT and mutated CD40-transfected HeLa cells. Cells were stimulated with 0.1 μg/ml CD40L or left untreated, as indicated. Nuclear proteins were isolated and examined for binding to a 32P-labeled oligonucleotide probe containing the HIV-LTR NF-κB binding site. Data were quantitated on a phosphorimager and expressed as the increase (n-fold) relative to unstimulated controls, which were given the arbitrary value of 1. The mean inducible increase (± standard deviation; n = 4 assays) in NF-κB binding was as follows: WT, 8.1 ± 1.6; CD40mT2/T3, 2.4 ± 0.3; CD40mT6, 5.7 ± 0.5; CD40mT2/T3/T6, 1.1 ± 0.05; and CD40mT3/T6, 5.3 ± 0.6. Supershift analysis using antibodies against the p65 and p50 NF-κB subunits confirmed that the DNA-bound proteins represent NF-κB (data not shown). Parallel cultures from a representative experiment were lysed and examined for IκBα or β-actin levels by immunoblotting.