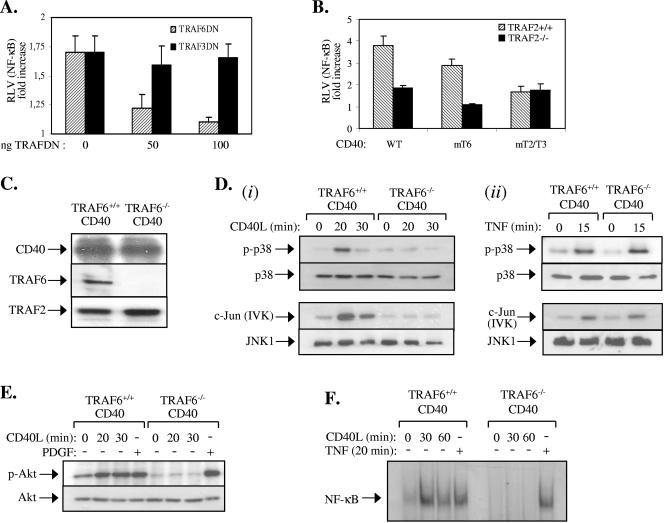
FIG. 6.
CD40 critically depends on TRAF6 for signal transduction. (A) A dominant-negative (DN), N terminus-deleted TRAF6 but not TRAF3 mutant suppresses CD40-mediated NF-κB transcriptional activity in the absence of TRAF2. Approximately 3 × 105 TRAF2−/− CD40 fibroblasts were transiently transfected with an NF-κB-responsive luciferase reporter and a β-galactosidase plasmid in the presence or absence of increasing amounts (0, 50, or 100 ng) of dominant-negative TRAFs. Thirty-six hours later, cells were stimulated with 0.5 μg/ml CD40L for 8 h or left untreated, and the luciferase and β-galactosidase activities were measured. The relative luciferase values (RLV) represent the increase (n-fold) in the ratio of the luciferase and β-galactosidase measurements relative to untreated controls, which were given the arbitrary value of 1. Data represent mean values ± standard deviations from three independent experiments. (B) The binding of TRAF6 to CD40 mediates NF-κB signaling in TRAF2-deficient cells. TRAF2+/+ CD40 or TRAF2−/− CD40 fibroblasts were transiently transfected with an NF-κB-responsive luciferase reporter and a β-galactosidase plasmid in the presence of WT CD40, CD40mT6, or CD40mT2/T3. Cells were then stimulated with CD40L and analyzed for reporter activity as described in panel A. Data represent mean values ± standard deviations from three independent experiments. (C) Immunoblot showing the expression of TRAF6, TRAF2, and CD40 in immortalized CD40-transfected TRAF6+/+ and TRAF6−/− fibroblasts used in this study. (D) Phosphorylation of p38 and activation of JNK are impaired in CD40L-stimulated (i) but not TNF-treated (ii) TRAF6−/− CD40 fibroblasts. TRAF6+/+ CD40 and TRAF6−/− CD40 fibroblasts were stimulated with CD40L for 20 or 30 min or left untreated. Lysates were immunoblotted for the phosphorylated or total p38 or immunoprecipitated with an anti-JNK1 antibody. The immunoprecipitates were examined for activity toward c-Jun as a substrate or were immunoblotted with a rabbit polyclonal JNK1 antibody. As a control, treatment with 25 ng/ml TNF induced similar levels of p38 and JNK activation in of TRAF6+/+ CD40 and TRAF6−/− CD40 fibroblasts. Data are representative of at least three independent experiments. IVK, in vitro kinase assay. (E) Phosphorylation of Akt is impaired in CD40L-stimulated but not PDGF-treated TRAF6−/− CD40 fibroblasts. TRAF6+/+ CD40 and TRAF6−/− CD40 fibroblasts were treated with 0.5 μg/ml CD40L for 20 or 30 min or with 50 ng/ml PDGF or were left untreated as indicated. Lysates were then immunoblotted for the phosphorylated or total Akt. (F) NF-κB binding activity is impaired in CD40L-stimulated but not TNF-treated TRAF6−/− CD40 fibroblasts. TRAF6+/+ CD40 and TRAF6−/− CD40 fibroblasts were treated with 0.5 μg/ml CD40L or 25 ng/ml TNF or were left untreated as indicated. Nuclear proteins were isolated and examined for binding to a 32P-labeled oligonucleotide probe containing the HIV-LTR NF-κB binding site. Three independent experiments were performed and gave similar results.