FIG. 2.
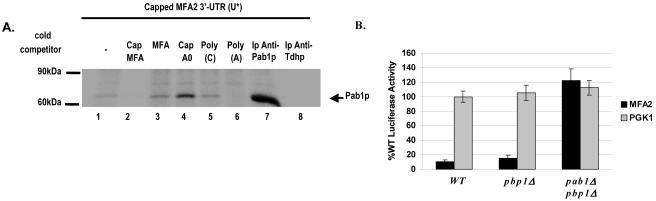
Pab1p binds the MFA2 3′-UTR in a cap-dependent manner and mediates translation repression. A. UV-cross-linking analysis performed with [α-32P]UTP-labeled capped MFA2 3′-UTR (U*) and wild-type yeast extracts. Competition was with a 100-fold excess of unlabeled capped and uncapped MFA2 3′-UTR in lanes 2 and 3, respectively; a 100-fold excess of nonspecific unlabeled capped polylinker RNA (Cap A0) in lane 4; and 100 ng of poly(C) and poly(A) in lanes 5 and 6, respectively. Pab1p reproducibly binds more strongly in the presence of a nonspecific RNA competitor (compare lane 1 to lanes 3 to 5). Two sets of the cross-linked reaction mixtures were subjected to immunoprecipitation with anti-Pab1p (lane 7) and anti-Tdh (lane 8) antibodies. B. Translation in the wild-type, pbp1Δ, and pbp1Δ pab1Δ strains. Black bars, translation of the Luc-MFA2 reporter. Gray bars, translation of the PGK1 reporter. All values are compared to the Luc-PGK1 reporter activity in the WT strain and are normalized for RNA levels. The luciferase experiment was repeated three times.