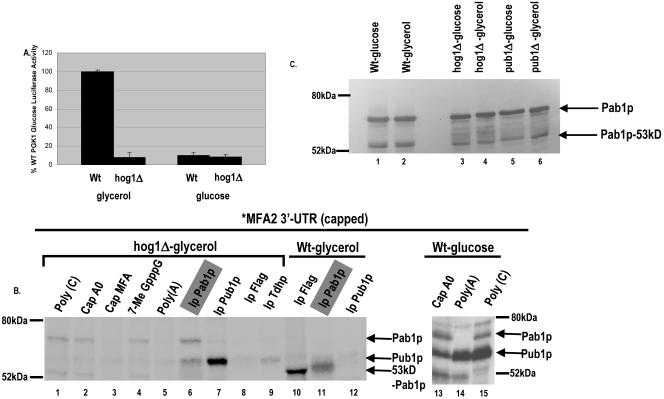
FIG. 4.
p38 MAPK/Hog1p controls carbon source-mediated ARE translation regulation. A. Translation in the hog1Δ strain grown in either glycerol or glucose medium. Shown is translation of the Luc-MFA2 reporter compared to the Luc-PGK1 reporter activity in the WT strain, normalized for RNA levels. The luciferase experiment is an average of three trials. B. UV-cross-linking analysis performed with [α-32P]UTP-labeled capped MFA2 3′-UTR (U*) and yeast extracts from the hog1Δ strain (lanes 1 to 9) or the wild-type strain grown in glycerol medium (lanes 10 to 12) or the wild-type strain grown in glucose medium (lanes 13 to 15). Shown is competition with a 100-fold excess of unlabeled cap in lane 4, a 100-fold excess of nonspecific unlabeled capped polylinker RNA (Cap A0) in lane 2 and lane 13, and 100 ng of poly(C) and poly(A) in lanes 1 and 5 and in lanes 15 and 14, respectively. Pab1p reproducibly binds more strongly in the presence of a nonspecific RNA competitor (compare lane 1 to lanes 3 to 5). Identical sets of the cross-linked reaction mixtures were subjected to immunoprecipitation with anti-Pab1p antibody (lane 6), anti-Pub1p antibody (lane 7), anti-Flag antibody (lane 8) as a monoclonal control antibody for the anti-Pub1p antibody, and anti-Tdh/GAPDH antibody (lane 9) as a polyclonal control antibody for the anti-Pab1p antibody. In wild-type glucose medium conditions, additional bands that are competed by poly(C) also appear that remain to be identified. C. Western blotting with anti-Pab1p polyclonal antibody with extracts used in this figure and Fig. 5 as marked.