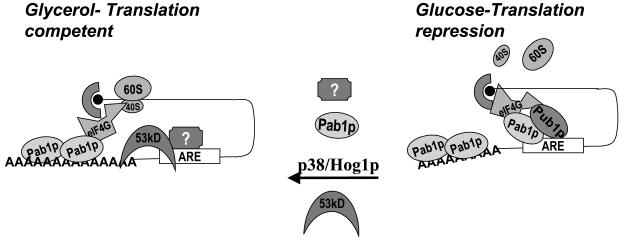
FIG. 6.
Pab1p mediates MFA2 translation regulation in a p38 MAPK/Hog1p-regulated pathway. p38 MAPK/Hog1p mediates translation regulation through the MFA2 3′-UTR in response to the presence of glucose medium growth conditions. In glucose-grown cultures, the 3′-UTR recruits Pub1p and Pab1p, which can interact with the eIF4G/cap complex as well. Pab1p may under these conditions be recruited to the AU element and interact with the eIF4G/cap complex. Such an mRNP would prevent the cap/poly(A) interaction that may be necessary to promote translation. Furthermore, the Pub1p-bound message may be localized to translationally silenced locations in the cell as described for the mammalian homolog TIA-1. Alternatively, in nonglucose conditions p38 MAPK/Hog1p may modulate Pub1p and Pab1p indirectly through unknown factors (represented as “?”) to prevent their recruitment to the MFA2 3′-UTR. The cap complex would not be involved in forming a translationally silenced complex through interactions with the 3′-UTR-3′-UTR-bound factors. This may be feasible due to the truncated form of Pab1p, the 53-kDa form, binding to the MFA2 3′-UTR instead of the full-length form (Pab1p). Pab1p may be modified such that the full-length form binds the poly(A) tail instead of the 3′-UTR, permitting a translationally favorable mRNP complex. Additionally, in the absence of Pub1p bound to the 3′-UTR, the transcript may be mobilized onto polysomes (depicted as 40S and 60S).