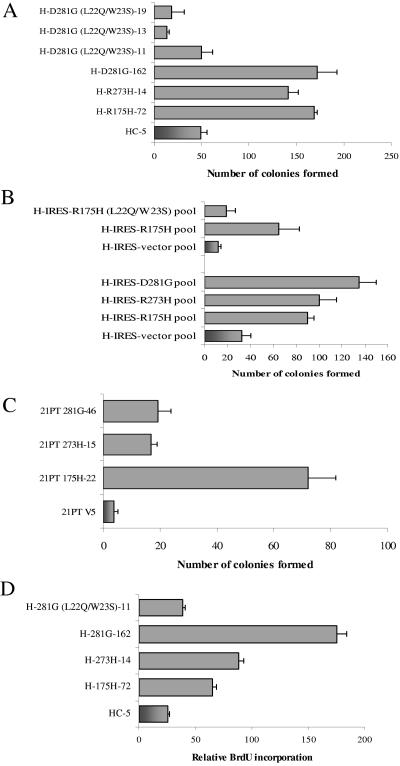
FIG. 1.
Expression of mutant p53 in H1299 and 21PT cells imparts decreased sensitivity to etoposide. (A to C) Cells were plated at 50,000 cells/plate and treated with 6 μM etoposide (final concentration) for 48 h. After treatment, cells were washed with Hanks balanced salt solution, and the surviving cells were allowed to recuperate for 3 weeks with periodic changes of medium. Colonies formed were fixed with methanol, stained with methylene blue, and counted. The data shown are representative of three independent experiments run simultaneously; colony numbers were adjusted to account for plating differences based on control plates. Control plates were plated at 1/10 the density and treated with DMSO for 48 h. Clones of H1299 cells expressing mutant p53-R175H, -R273H, -D281G, p53-D281G (L22Q/W23S), or vector alone (panel A), pools of H1299 cells expressing p53-R175H, -R175H (L22Q/W23S), -R273H, -D281G, or vector alone (panel B), and clones of 21PT cells expressing mutant p53-R175H, -R273H, -D281G, or vector alone (panel C) were used. (D) H1299 cells expressing mutant p53-R175H, -R273H, -D281G, the transactivation-deficient p53-D281G (L22Q/W23S), or vector alone were exposed to 6 μM etoposide (final concentration) for 48 h. After treatment, cells were allowed to incorporate BrdU for 40 min, washed, and fixed. Prior to florescence-activated cell sorting analysis, cells were stained with a fluorescein isothiocyanate-coupled anti-BrdU antibody and propidium iodide as described previously (32).