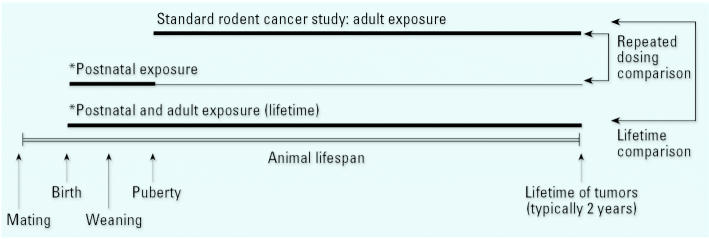
Figure 1.
Schematic representation of several cancer study designs reported in the evaluated literature. The standard rodent bioassay begins after puberty, and exposures continue for about 2 years. Repeated-dosing studies typically dose during the postnatal period, with observations for tumors at approximately 2 years. Lifetime studies combine postnatal and adult exposures, sometimes beginning with in utero exposure. Acute studies (not shown) generally involve one or a few exposure during the in utero, preweaning, prepubertal, and adult periods. The adult tumors were often evaluated much earlier than 2 years
*Can also include prenatal exposure.