Abstract
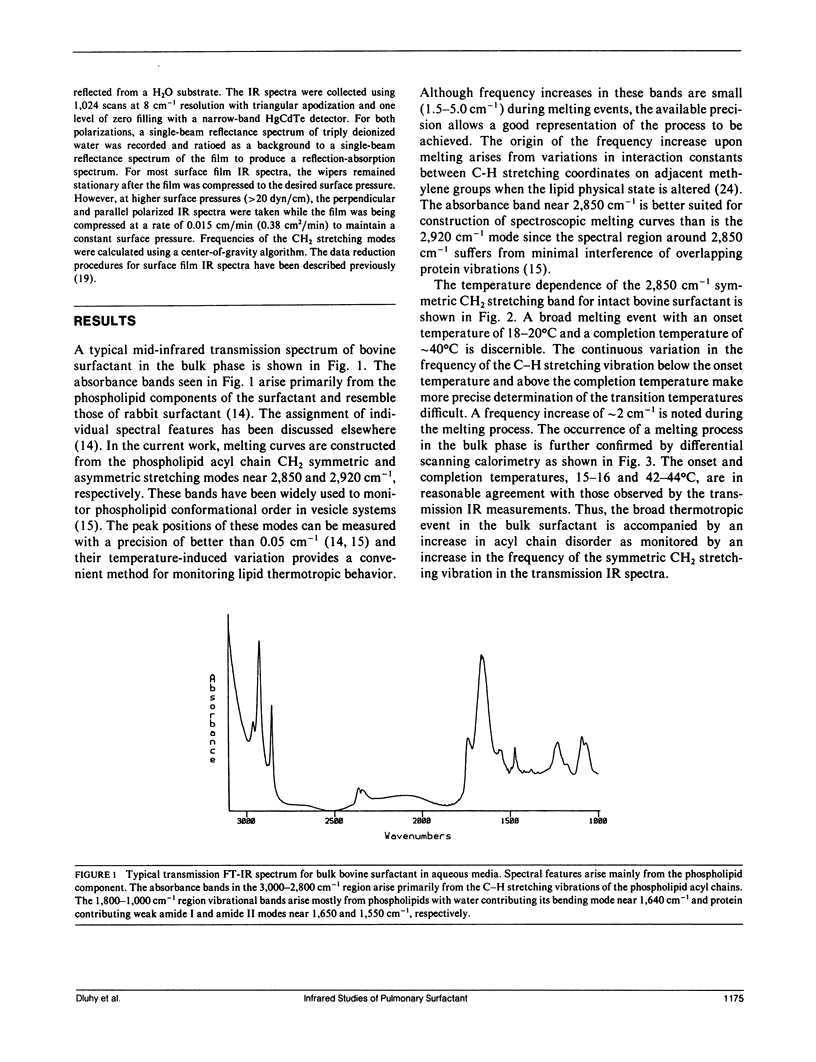
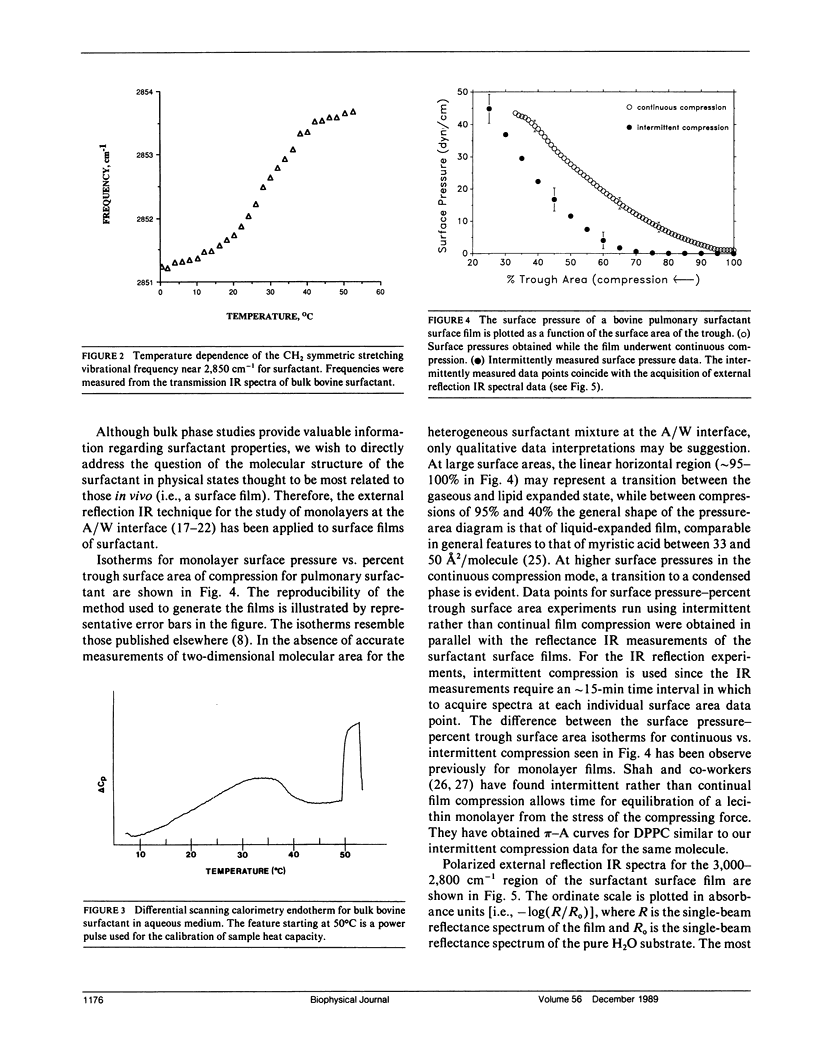
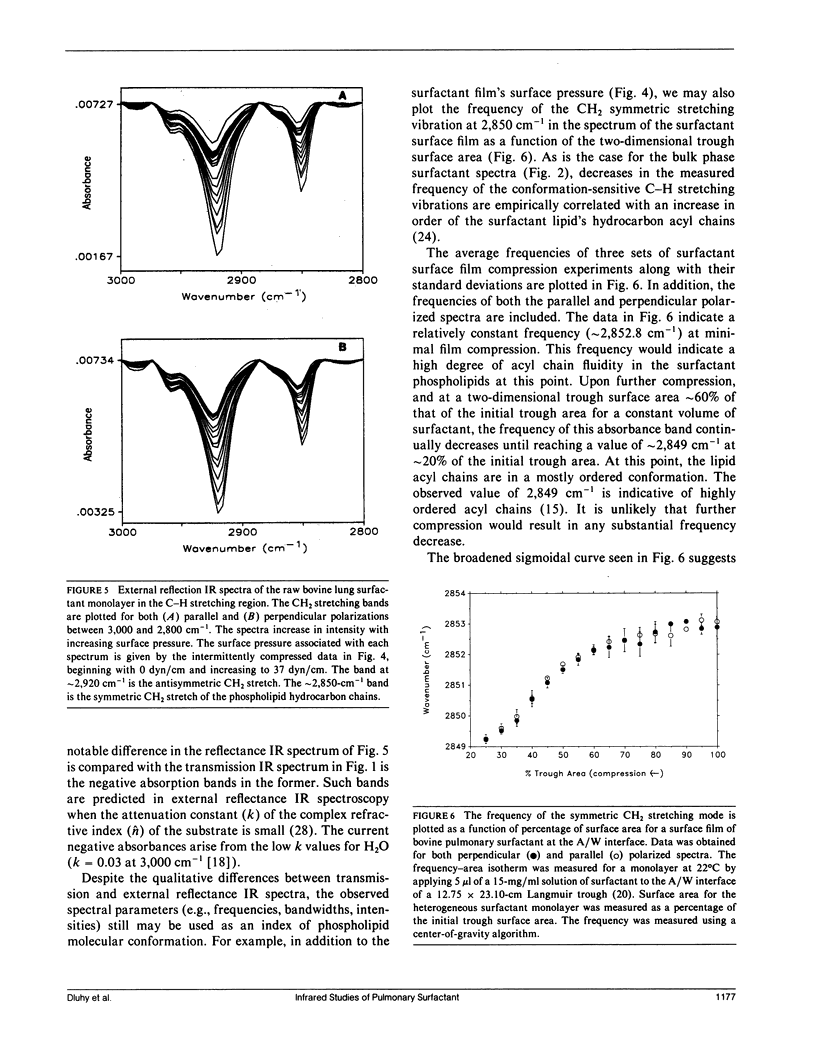
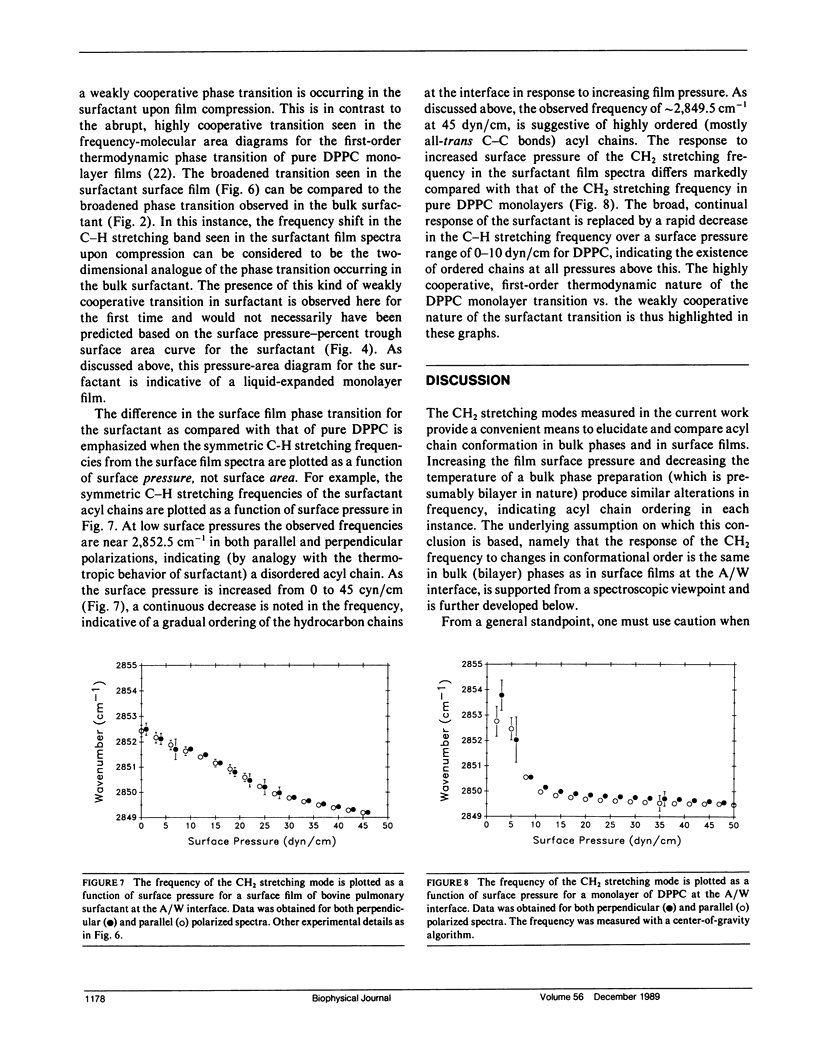
The molecular structure of the phospholipid component of intact pulmonary surfactant isolated from bovine lung lavage has been examined by Fourier transform infrared spectroscopy. Two different physical states of the surfactant were examined by means of different infrared spectroscopic sampling techniques. Transmission infrared experiments were used to study the surfactant in the bulk phase. In these experiments, the thermotropic behavior of the bulk surfactant was monitored by temperature-induced variations in the phospholipid acyl chain CH2 stretching frequencies. A broad phase transition (confirmed by differential scanning calorimetry) was noted with an onset temperature near 15 degrees C and a completion temperature near 42 degrees C. In addition to the bulk transmission experiments, external reflection infrared spectroscopy was used to examine surfactant films in situ at the air-water interface. As surface pressure was increased from 0 to 43 dyn/cm, a gradual and continuous decrease in the CH2 stretching frequency was noted for the surfactant. Thus, under surface pressures which correspond to large lung volumes in vivo, the surfactant acyl chains exist mostly in the ordered (trans) configuration. The frequency shift in the CH2 stretching mode is consistent with a continuous ordering of the acyl chains upon compression over the pressure range 0-43 dyn/cm, and implies that a weakly cooperative phase transition occurs in the hydrocarbon region of the surface film. The surface film transition is especially noted in the pressure-area curve of the surfactant and approximates in two dimensions the broad thermotropic phase transition of the bulk phase surfactant. Substantial differences were observed between the response to surface pressure changes of intact surfactant compared with the main surfactant phospholipid, 1,2-dipalmitoyl-sn--glycero-3-phosphocholine. The changes in response are attributed to the presence of additional surfactant components. The current work demonstrates the ability of infrared spectroscopy to obtain structural information on the surfactant in physical states that directly relate to those in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clements J. A. Functions of the alveolar lining. Am Rev Respir Dis. 1977 Jun;115(6 Pt 2):67–71. doi: 10.1164/arrd.1977.115.S.67. [DOI] [PubMed] [Google Scholar]
- Colacicco G., Basu M. K., Scarpelli E. M. pH, temperature, humidity and the dynamic force-area curve of dipalmitoyl lecithin. Respir Physiol. 1976 Aug;27(2):169–186. doi: 10.1016/0034-5687(76)90072-4. [DOI] [PubMed] [Google Scholar]
- Galdston M., Shah D. O. Surface properties and hysteresis of dipalmitoyllecithin in relation to the alveolar lining layer. Biochim Biophys Acta. 1967 Apr 4;137(2):255–263. doi: 10.1016/0005-2760(67)90101-4. [DOI] [PubMed] [Google Scholar]
- Hawco M. W., Coolbear K. P., Davis P. J., Keough K. M. Exclusion of fluid lipid during compression of monolayers of mixtures of dipalmitoylphosphatidylcholine with some other phosphatidylcholines. Biochim Biophys Acta. 1981 Aug 6;646(1):185–187. doi: 10.1016/0005-2736(81)90286-8. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Hamilton R. L., Jr Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry. 1985 Jan 1;24(1):184–190. doi: 10.1021/bi00322a026. [DOI] [PubMed] [Google Scholar]
- Hook G. E., Spalding J. W., Ortner M. J., Tombropoulos E. G., Chignell C. F. Investigation of phospholipids of the pulmonary extracellular lining by electron paramagnetic resonance. The effects of phosphatidylglycerol and unsaturated phosphatidylcholines on the fluidity of dipalmitoyl phosphatidylcholine. Biochem J. 1984 Oct 15;223(2):533–542. doi: 10.1042/bj2230533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough K. M., Farrell E., Cox M., Harrell G., Taeusch H. W., Jr Physical, chemical, and physiological characteristics of isolates of pulmonary surfactant from adult rabbits. Can J Physiol Pharmacol. 1985 Sep;63(9):1043–1051. doi: 10.1139/y85-171. [DOI] [PubMed] [Google Scholar]
- King R. J., MacBeth M. C. Interaction of the lipid and protein components of pulmonary surfactant. Role of phosphatidylglycerol and calcium. Biochim Biophys Acta. 1981 Oct 2;647(2):159–168. doi: 10.1016/0005-2736(81)90242-x. [DOI] [PubMed] [Google Scholar]
- King R. J., Macbeth M. C. Physicochemical properties of dipalmitoyl phosphatidylcholine after interaction with an apolipoprotein of pulmonary surfactant. Biochim Biophys Acta. 1979 Oct 19;557(1):86–101. doi: 10.1016/0005-2736(79)90092-0. [DOI] [PubMed] [Google Scholar]
- Magoon M. W., Wright J. R., Baritussio A., Williams M. C., Goerke J., Benson B. J., Hamilton R. L., Clements J. A. Subfractionation of lung surfactant. Implications for metabolism and surface activity. Biochim Biophys Acta. 1983 Jan 7;750(1):18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- Mautone A. J., Reilly K. E., Mendelsohn R. Fourier transform infrared and differential scanning calorimetric studies of a surface-active material from rabbit lung. Biochim Biophys Acta. 1987 Jan 9;896(1):1–10. doi: 10.1016/0005-2736(87)90349-x. [DOI] [PubMed] [Google Scholar]
- SHAH D. O., SCHULMAN J. H. BINDING OF METAL IONS TO MONOLAYERS OF LECITHINS, PLASMALOGEN, CARDIOLIPIN, AND DICETYL PHOSPHATE. J Lipid Res. 1965 Jul;6:341–349. [PubMed] [Google Scholar]
- Schürch S., Bachofen H., Weibel E. R. Alveolar surface tensions in excised rabbit lungs: effect of temperature. Respir Physiol. 1985 Oct;62(1):31–45. doi: 10.1016/0034-5687(85)90048-9. [DOI] [PubMed] [Google Scholar]
- Schürch S. Surface tension at low lung volumes: dependence on time and alveolar size. Respir Physiol. 1982 Jun;48(3):339–355. doi: 10.1016/0034-5687(82)90038-x. [DOI] [PubMed] [Google Scholar]
- Shiffer K., Hawgood S., Düzgünes N., Goerke J. Interactions of the low molecular weight group of surfactant-associated proteins (SP 5-18) with pulmonary surfactant lipids. Biochemistry. 1988 Apr 19;27(8):2689–2695. doi: 10.1021/bi00408a008. [DOI] [PubMed] [Google Scholar]
- Taylor M. G., Smith I. C. The fidelity of response by nitroxide spin probes to changes in membrane organization: the condensing effect of cholesterol. Biochim Biophys Acta. 1980 Jun 20;599(1):140–149. doi: 10.1016/0005-2736(80)90063-2. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Benson B. J., Williams M. C., Goerke J., Clements J. A. Protein composition of rabbit alveolar surfactant subfractions. Biochim Biophys Acta. 1984 Dec 21;791(3):320–332. doi: 10.1016/0167-4838(84)90343-1. [DOI] [PubMed] [Google Scholar]
- van DEENEN L., HOUTSMULLERUM, de HASS G., MULDER E. Monomolecular layers of synthetic phosphatides. J Pharm Pharmacol. 1962 Jul;14:429–444. doi: 10.1111/j.2042-7158.1962.tb11121.x. [DOI] [PubMed] [Google Scholar]


