Abstract
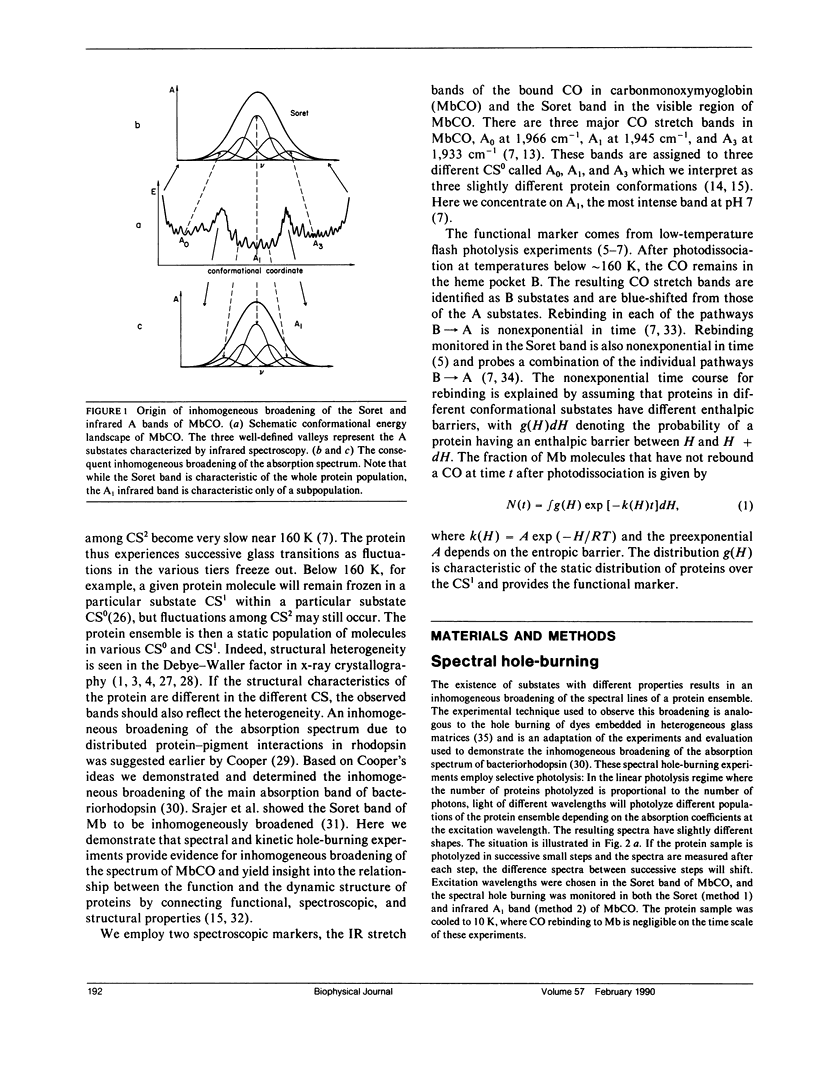
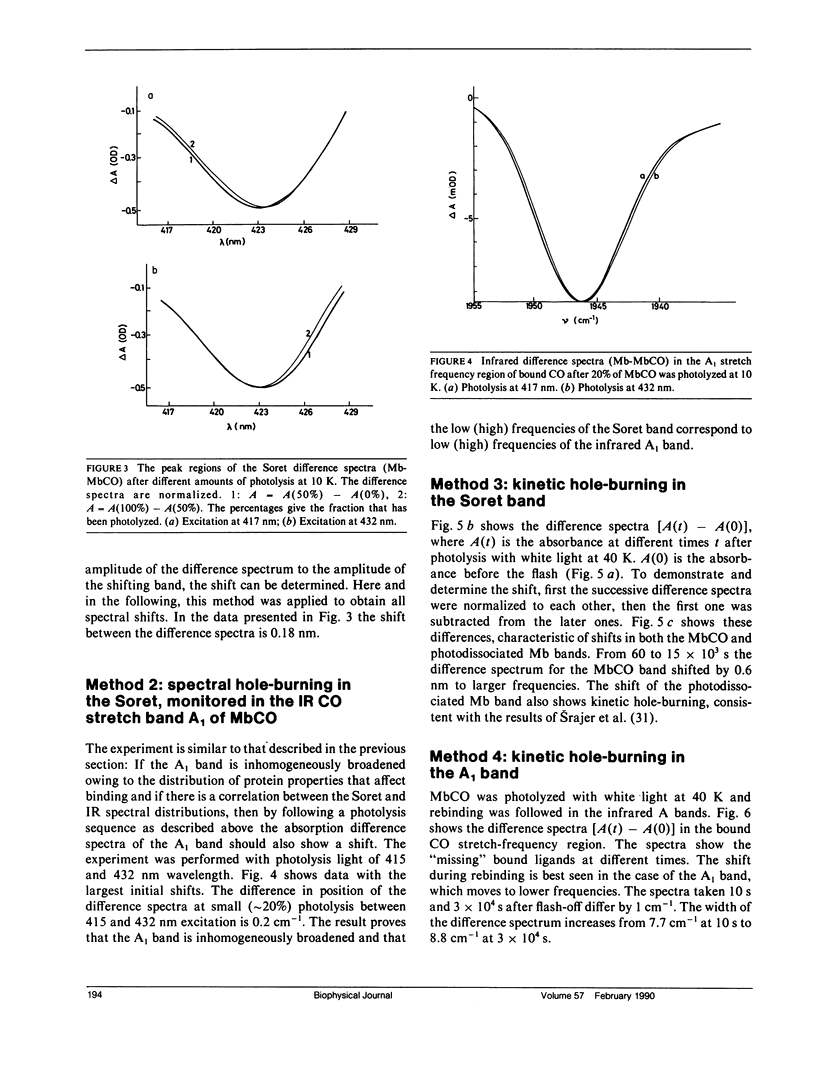
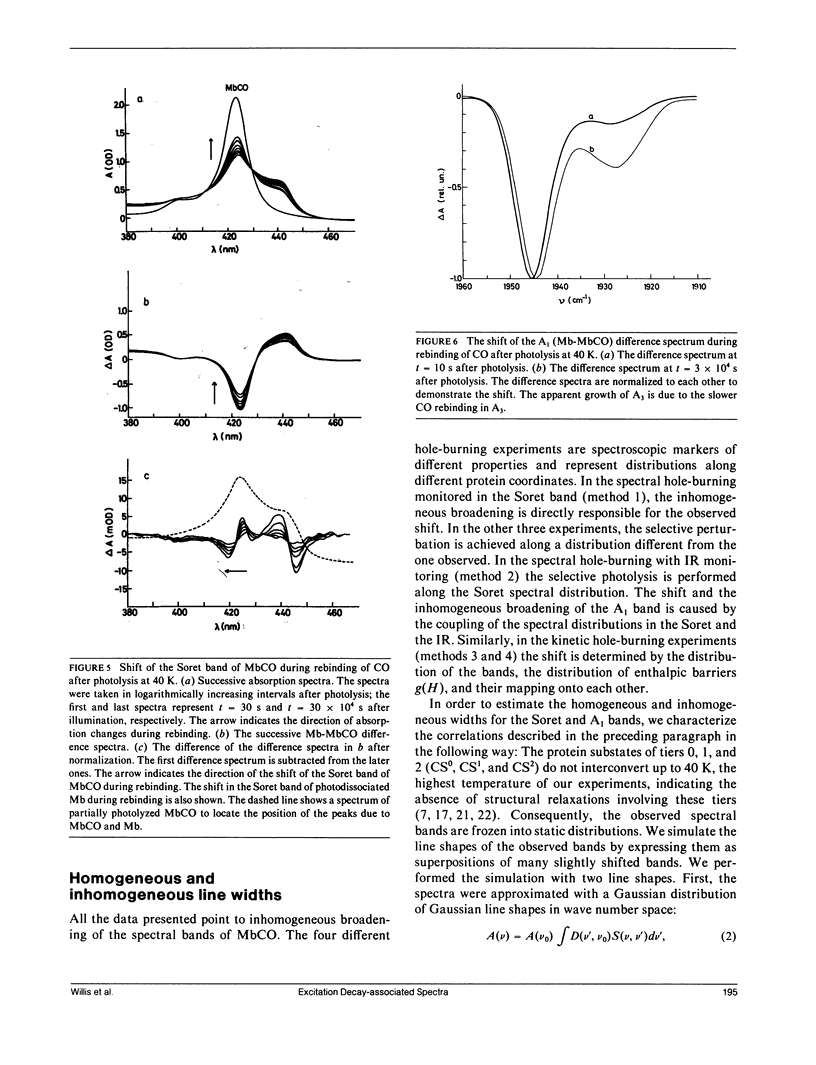
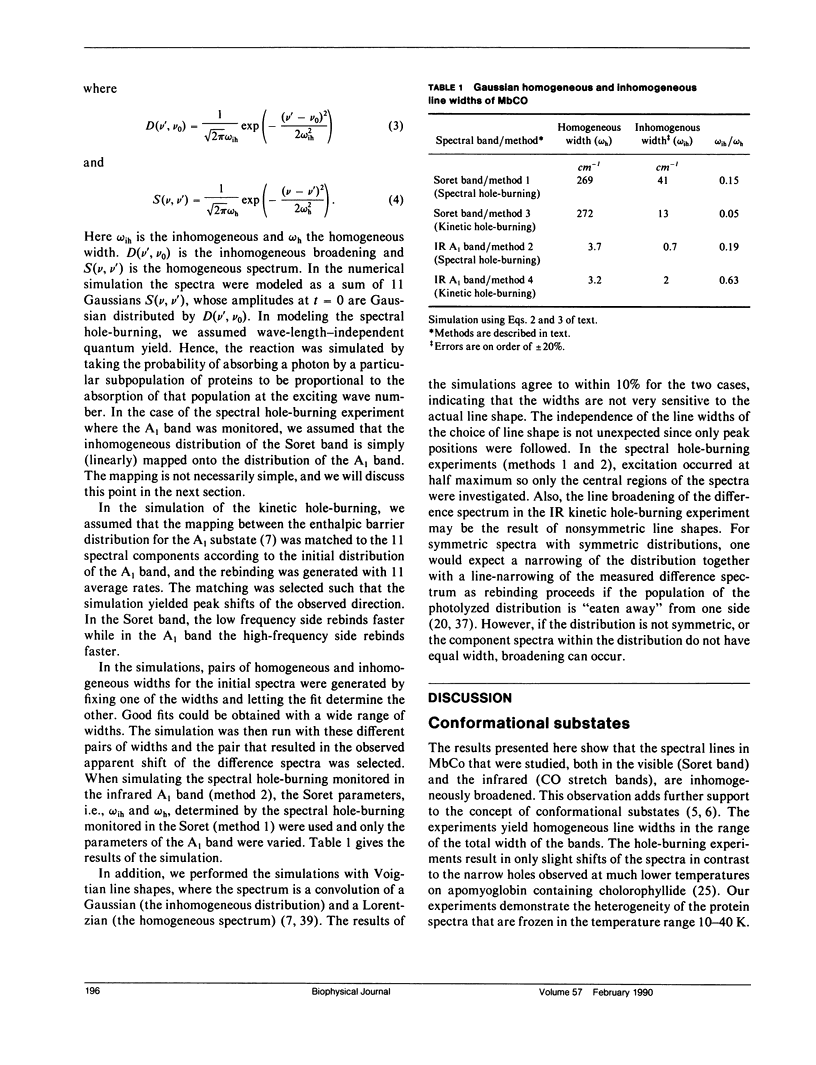
The rebinding kinetics of CO to myoglobin after flash photolysis is nonexponential in time below approximately 180 K; the kinetics is governed by a distribution of enthalpic barriers. This distribution results from inhomogeneities in the protein conformation, referred to as conformational substates. Hole-burning experiments on the Soret and IR CO-stretch bands test the assumption that an inhomogeneous distribution of conformational substates results in inhomogeneously broadened spectra. CO was slowly photolyzed at different wavelengths in the Soret band at 10 K. Both the Soret band and the CO-stretch band A1, centered at 1,945 cm-1, shift during photolysis, demonstrating that different wavelengths excite different parts of the distributed population. We have also done kinetic hole-burning experiments by measuring peak shifts in the Soret and A1 bands as the CO molecules rebind. The shifts indicate that the spectral and enthalpic distributions are correlated. In the A1 band, the spectral and enthalpic distributions are highly correlated while in the Soret the correlation is weak. From the peak shifts in the spectral and kinetic hole-burning experiments the inhomogeneous broadening is estimated to be approximately 15% of the total width in the Soret band and approximately 60% in A1. We have previously measured the tilt angle alpha between the bound CO and the heme normal (Ormos, P., D. Braunstein, H. Frauenfelder, M. K. Hong, S.-L. Lin, T. B. Sauke, and R. D. Young. 1988. Proc. Natl. Acad. Sci. USA. 85:8492-8496) and observed a wave number dependence of the tilt angles within the CO-stretch A bands. Thus the spectral and enthalpic distributions of the A bands are coupled to a heterogeneity of the structure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agmon N. Reactive line-shape narrowing in low-temperature inhomogeneous geminate recombination of CO to myoglobin. Biochemistry. 1988 May 3;27(9):3507–3511. doi: 10.1021/bi00409a057. [DOI] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Linkage of functional and structural heterogeneity in proteins: dynamic hole burning in carboxymyoglobin. Science. 1987 Oct 16;238(4825):373–376. doi: 10.1126/science.3659921. [DOI] [PubMed] [Google Scholar]
- Chance M. R., Campbell B. F., Hoover R., Friedman J. M. Myoglobin recombination at low temperature. Two phases revealed by Fourier transform infrared spectroscopy. J Biol Chem. 1987 May 25;262(15):6959–6961. [PubMed] [Google Scholar]
- Cooper A. Conformational fluctuation and change in biological macromolecules. Sci Prog. 1980;66(264):473–497. [PubMed] [Google Scholar]
- Coughey W. S., Alben J. O., McCoy S., Boyer S. H., Charache S., Hathaway P. Differences in the infrared stretching frequency of carbon monoxide bound to abnormal hemoglobins. Biochemistry. 1969 Jan;8(1):59–62. doi: 10.1021/bi00829a009. [DOI] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Parak F., Steigemann W., Petsko G. A., Ponzi D. R., Frauenfelder H. Conformational substates in a protein: structure and dynamics of metmyoglobin at 80 K. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4967–4971. doi: 10.1073/pnas.79.16.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iben IE, Braunstein D, Doster W, Frauenfelder H, Hong MK, Johnson JB, Luck S, Ormos P, Schulte A, Steinbach PJ. Glassy behavior of a protein. Phys Rev Lett. 1989 Apr 17;62(16):1916–1919. doi: 10.1103/PhysRevLett.62.1916. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Houtchens R. A., Caughey W. S. Structure of carboxymyoglobin in crystals and in solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6042–6046. doi: 10.1073/pnas.76.12.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. N., Hansen P. A., Hochstrasser R. M. Iron-carbonyl bond geometries of carboxymyoglobin and carboxyhemoglobin in solution determined by picosecond time-resolved infrared spectroscopy. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5062–5066. doi: 10.1073/pnas.85.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormos P., Braunstein D., Frauenfelder H., Hong M. K., Lin S. L., Sauke T. B., Young R. D. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8492–8496. doi: 10.1073/pnas.85.22.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parak F., Knapp E. W. A consistent picture of protein dynamics. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7088–7092. doi: 10.1073/pnas.81.22.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsko G. A., Ringe D. Fluctuations in protein structure from X-ray diffraction. Annu Rev Biophys Bioeng. 1984;13:331–371. doi: 10.1146/annurev.bb.13.060184.001555. [DOI] [PubMed] [Google Scholar]
- Srajer V, V, Schomacker KT, Champion PM. Spectral broadening in biomolecules. Phys Rev Lett. 1986 Sep 8;57(10):1267–1270. doi: 10.1103/PhysRevLett.57.1267. [DOI] [PubMed] [Google Scholar]


