Abstract
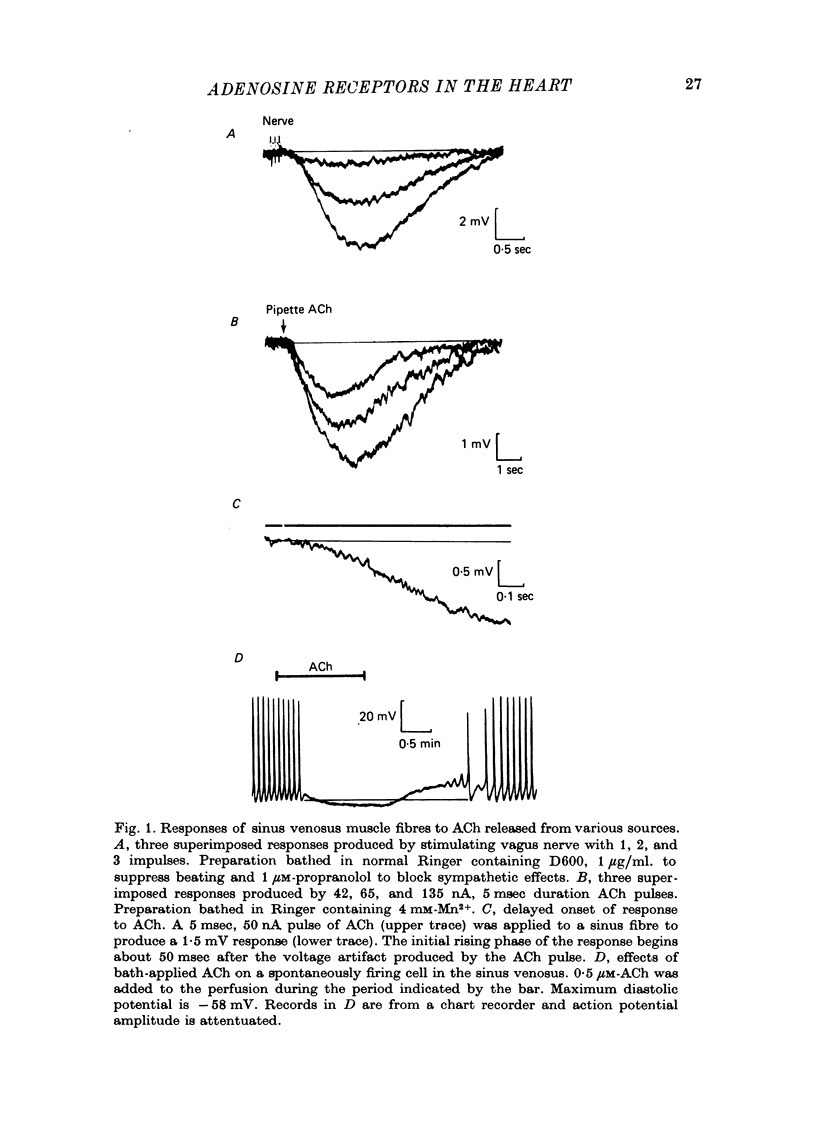
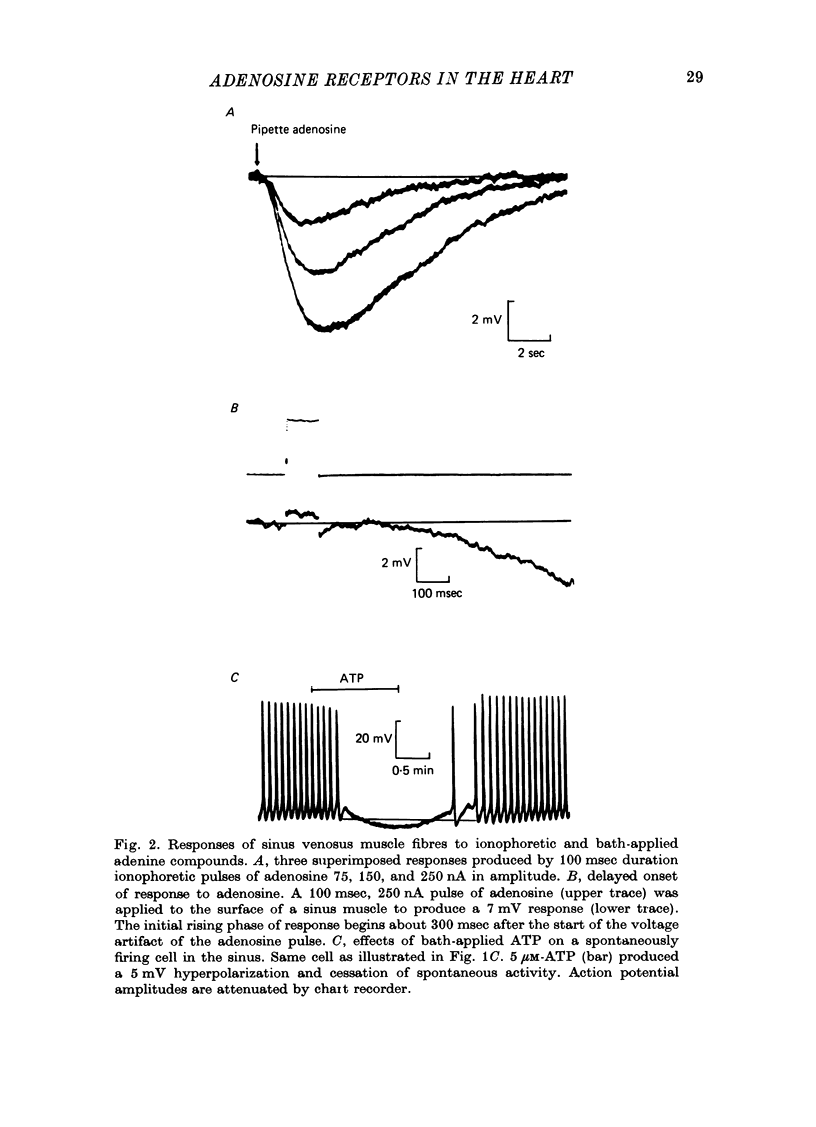
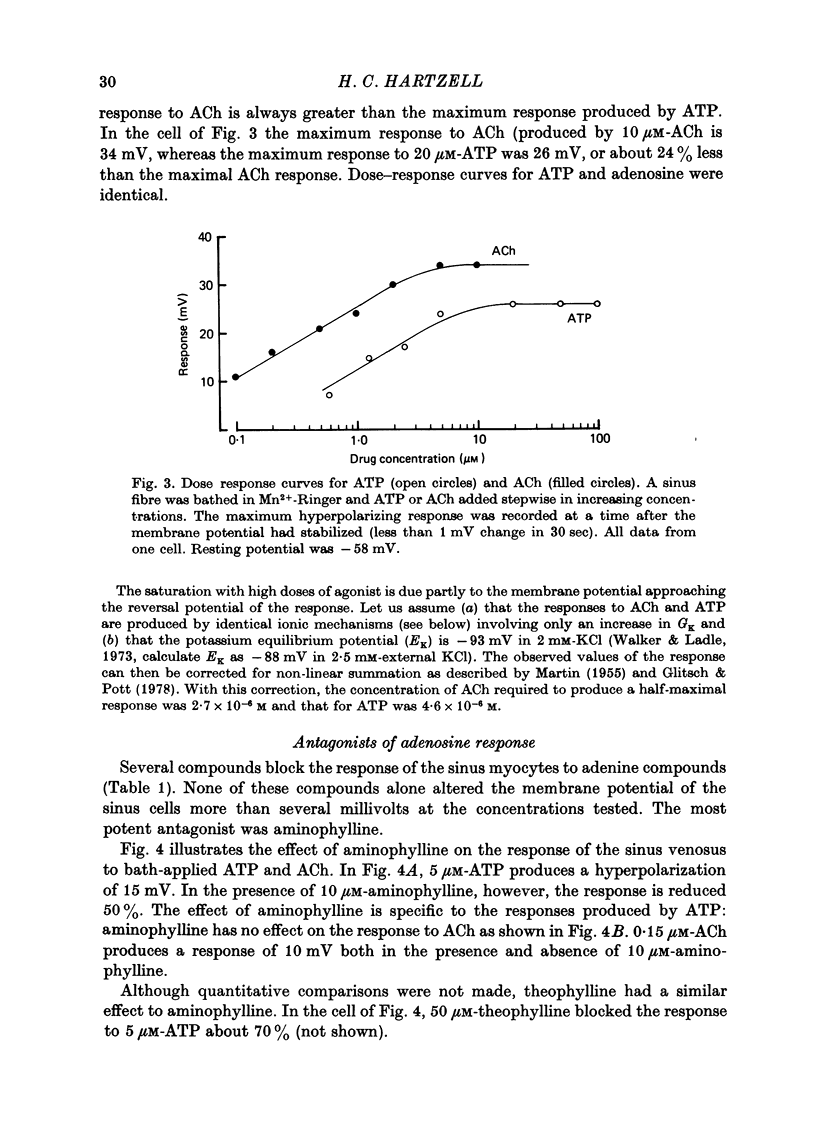
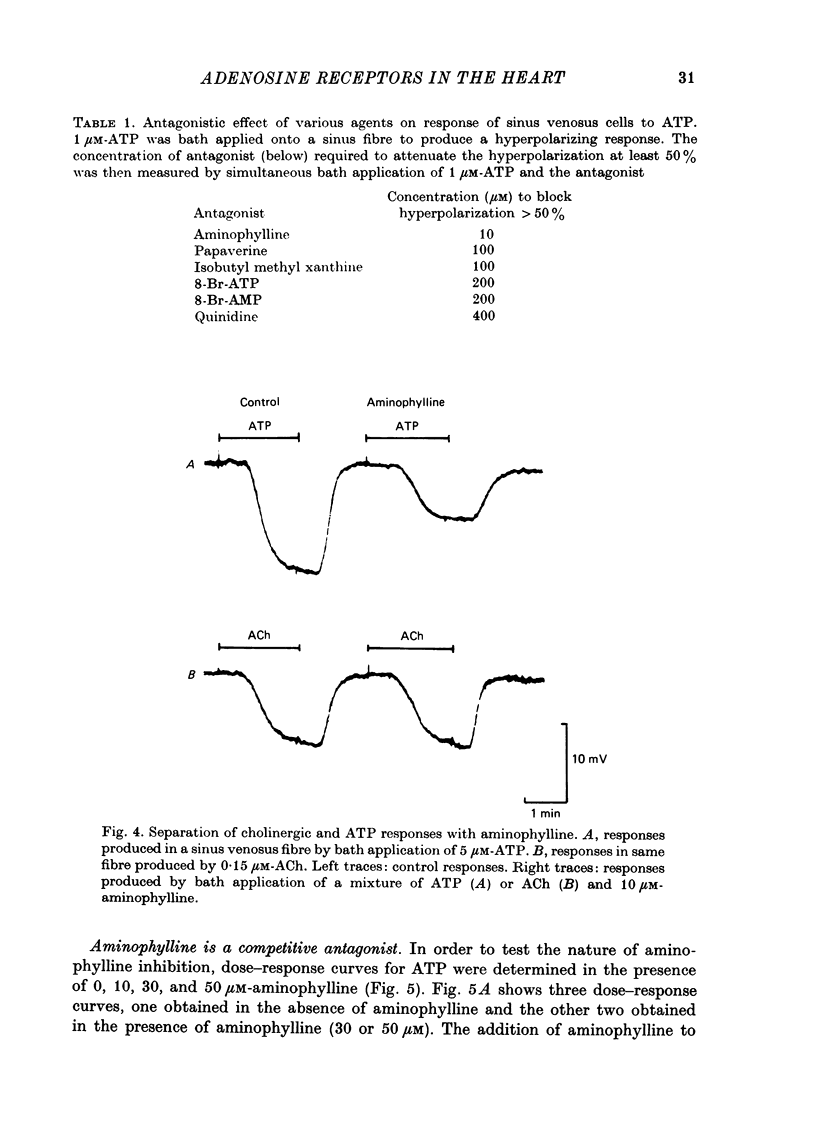
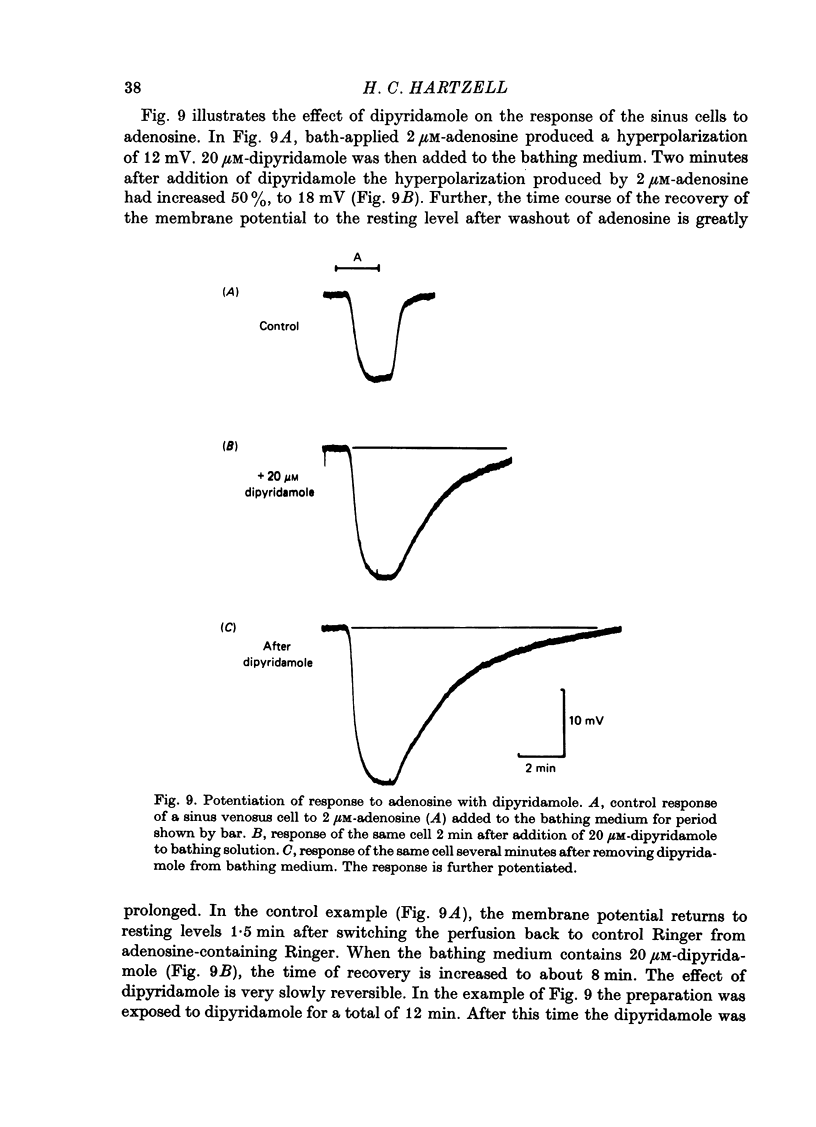
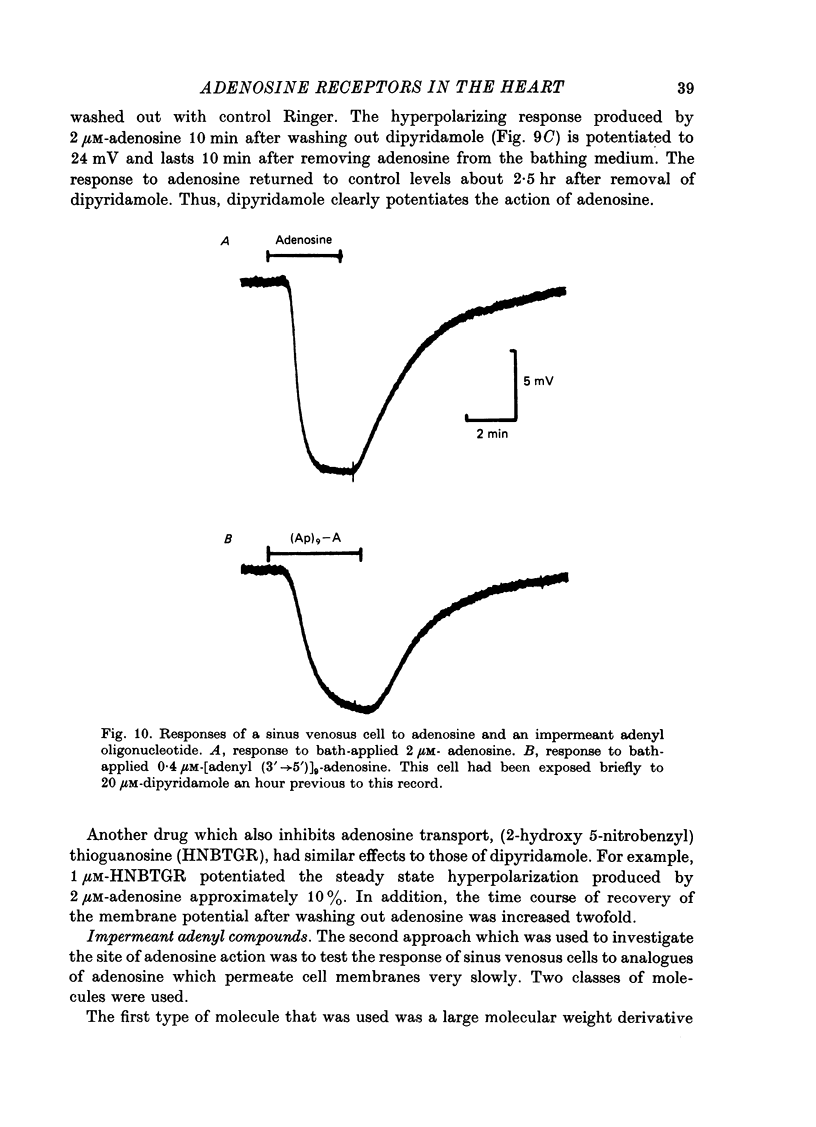
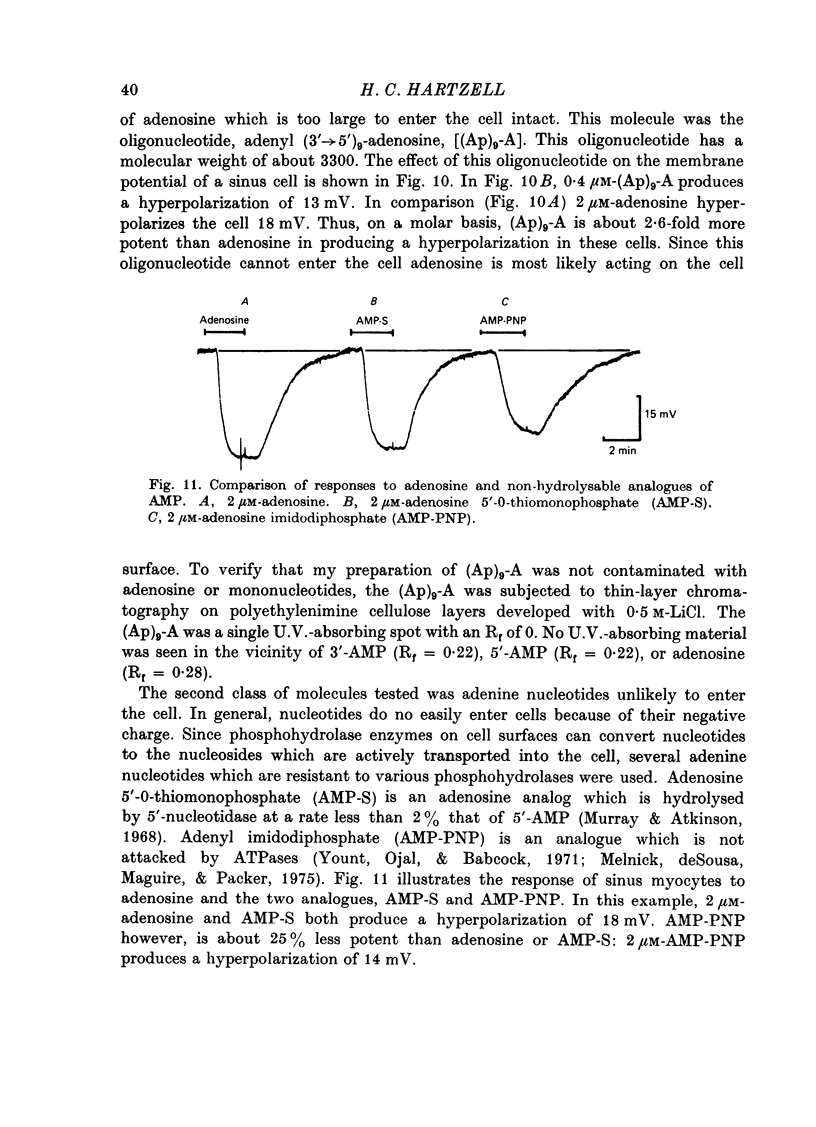
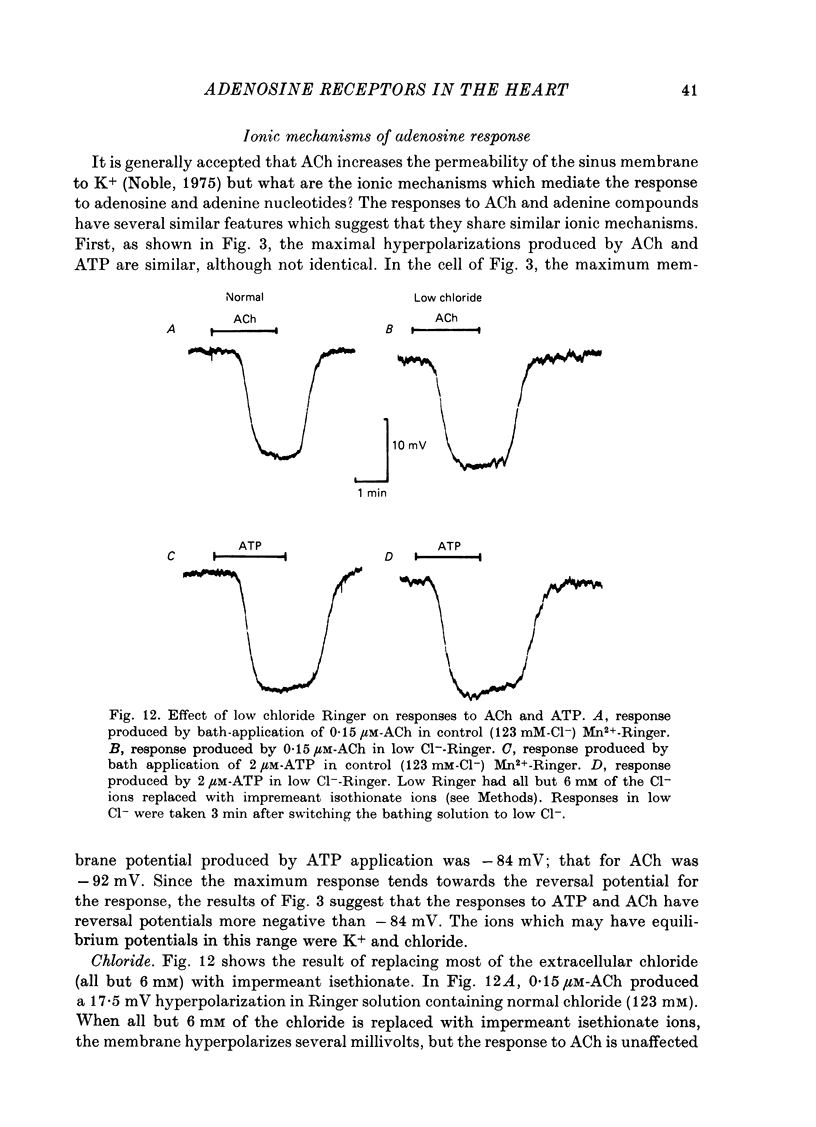
1. Membrane potential changes produced by adenosine and adenine nucleotides, acetylcholine, and vagus nerve stimulation were studied by intracellular recording in the sinus venosus of the frog, Rana pipiens. 2. Acetylcholine (ACh) released from the vagus nerve terminals evoked a slow hyperpolarization lasting several seconds in the cells of the sinus. Ionophoretic application of ACh from a micropipette produced a response which is similar in time course and amplitude to that evoked by vagus nerve stimulation. Bath application of ACh caused a steady hyperpolarization in quiescent preparations, or cessation of action potential generation in spontaneously active preparations. 3. Adenosine and adenine nucleotides produced hyperpolarizations when applied by addition to the bath or by ionophoresis from micropipettes. The hyperpolarization produced by ionophoresis of adenine compounds was somewhat slower than that produced by ACh. 4. Adenosine and the adenine nucleotides, 5'-AMP, 3'-AMP, 2'-AMP, and 5'-atp were virtually equipotent in their action. Adenosine was at least 1000-fold more potent than other purine and pyrimidine nucleosides or adenine. Both the ribose and adenine groups were important for agonist activity. 5. The concentrations of agonist required to produce half-maximal responses were estimated from dose--response curves as 3 x 10(-7) M for ACh and 2 x 10(-6) M for ATP. ACh is about 7 times more potent than ATP in producing a hyperpolarization. 6. Adenine compounds act directly upon the cardiac muscle fibres: bath or ionophoretically applied adenine compounds act even when transmitter release from nerve terminals is blocked with high (Mn2+) or when ACh receptors are blocked with atropine. 7. Adenine compounds act on the surface of the muscle fibre membrane. Analogues of adenosine which do not enter the cell are potent agonists of the receptor. An adenyl oligonucleotide too large to enter the cell was 2.6 times more potent per mole than adenosine in producing a hyperpolarization. Drugs such as dipyridamole and 6-(2-hydroxy 5-nitrobenzyl) thioguanosine, which are potent blockers of adenosine transport, potentiate the response of the sinus cells to adenosine. 8. Aminophylline and theophylline are competitive antagonists of adenosine action. The apparent Ki for aminophylline inhibition was 5 microM. 9. The response produced by adenine compounds is partly caused by an increase in the permeability of the membrane to K+. The maximum response to both ACh and adenine nucleotides approached the estimated level of EK or ECl. Replacing extracellular chloride with impermeant isethionate had no effect on responses to ACh or adenine nucleotides. The hyperpolarization was not produced by an activation of an ouabain-sensitive pump since 20 microM-ouabain had little effect on the response to adenosine. 10. The response to vagus nerve stimulation is completely blocked by 50 nM-atropine...
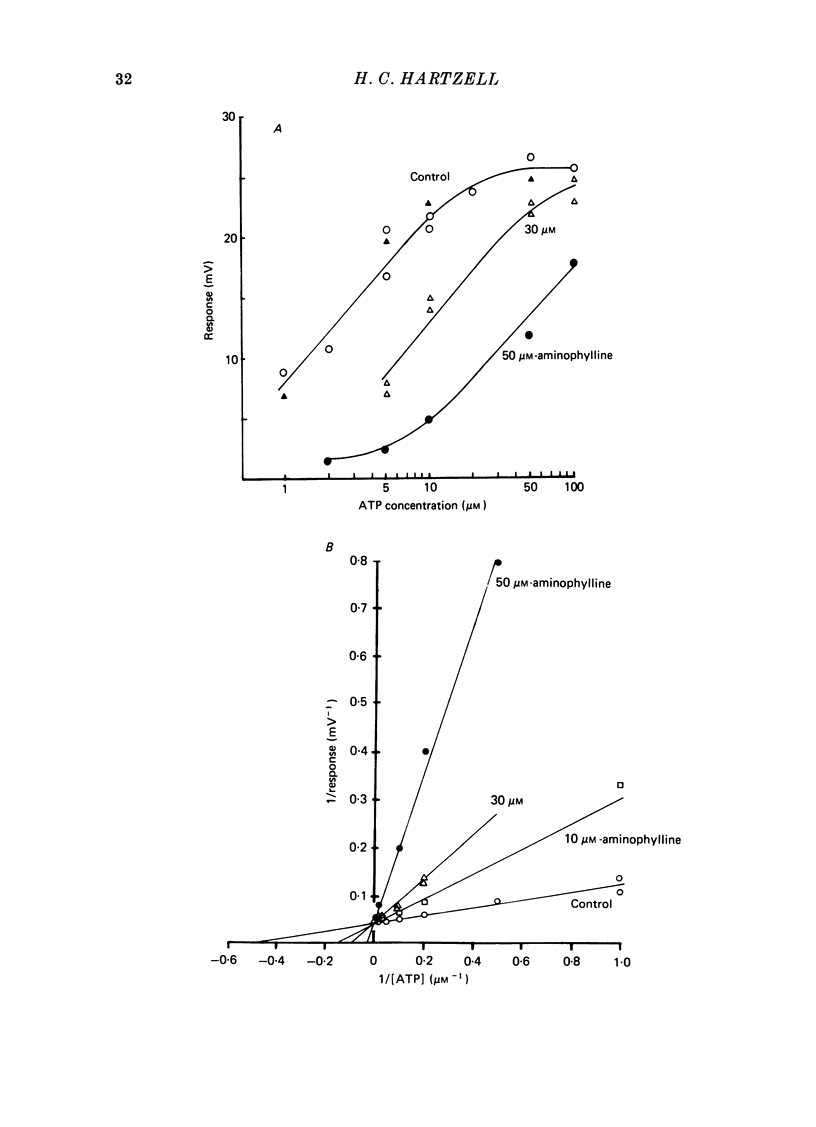
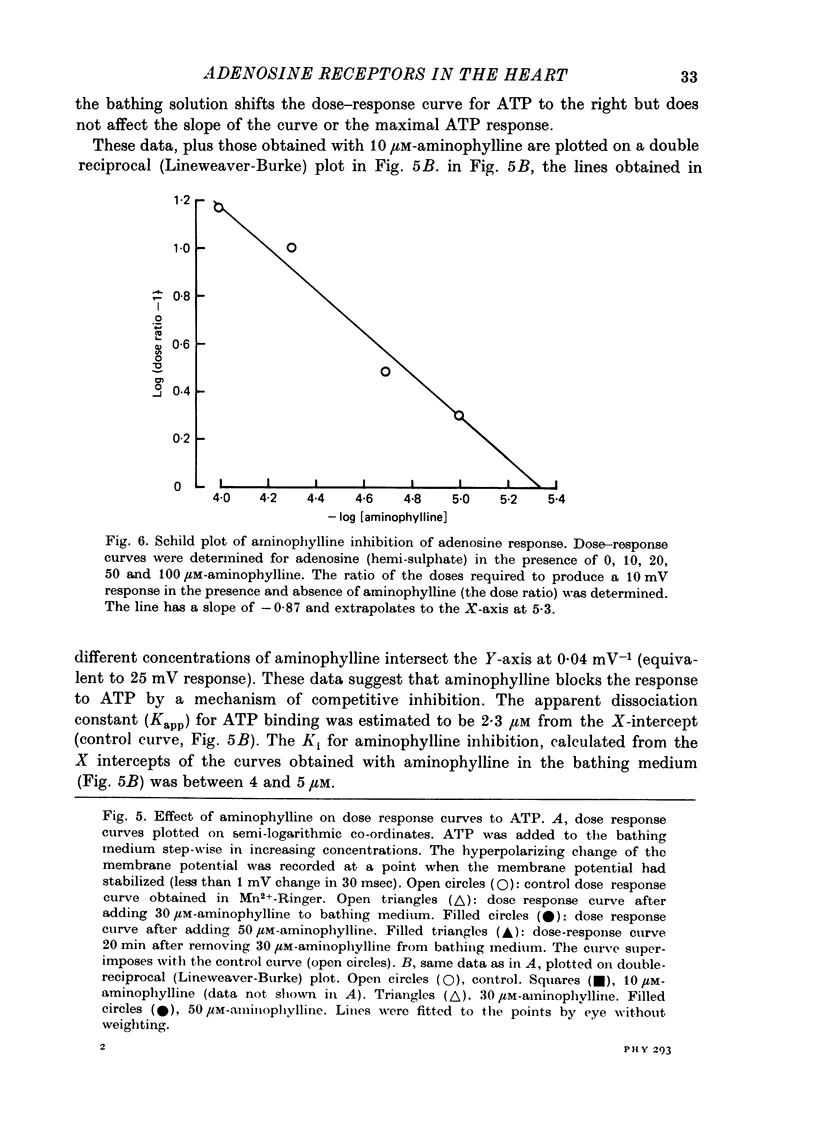
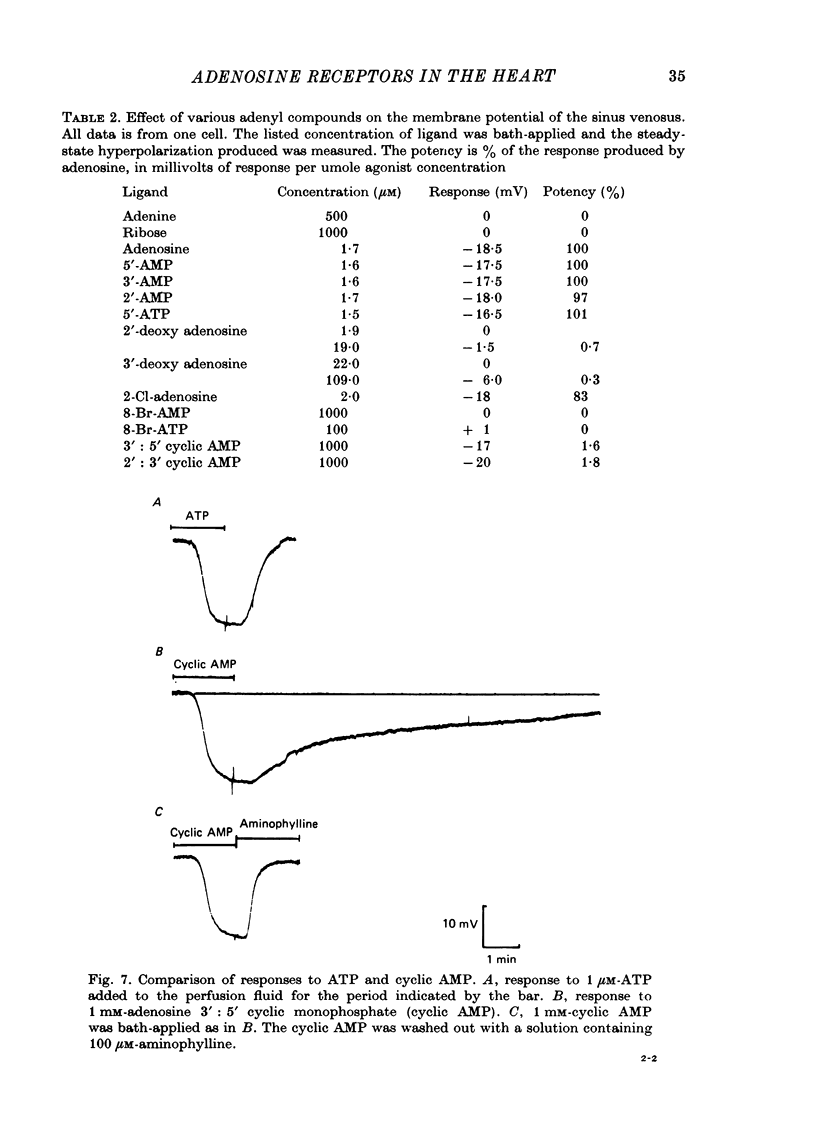
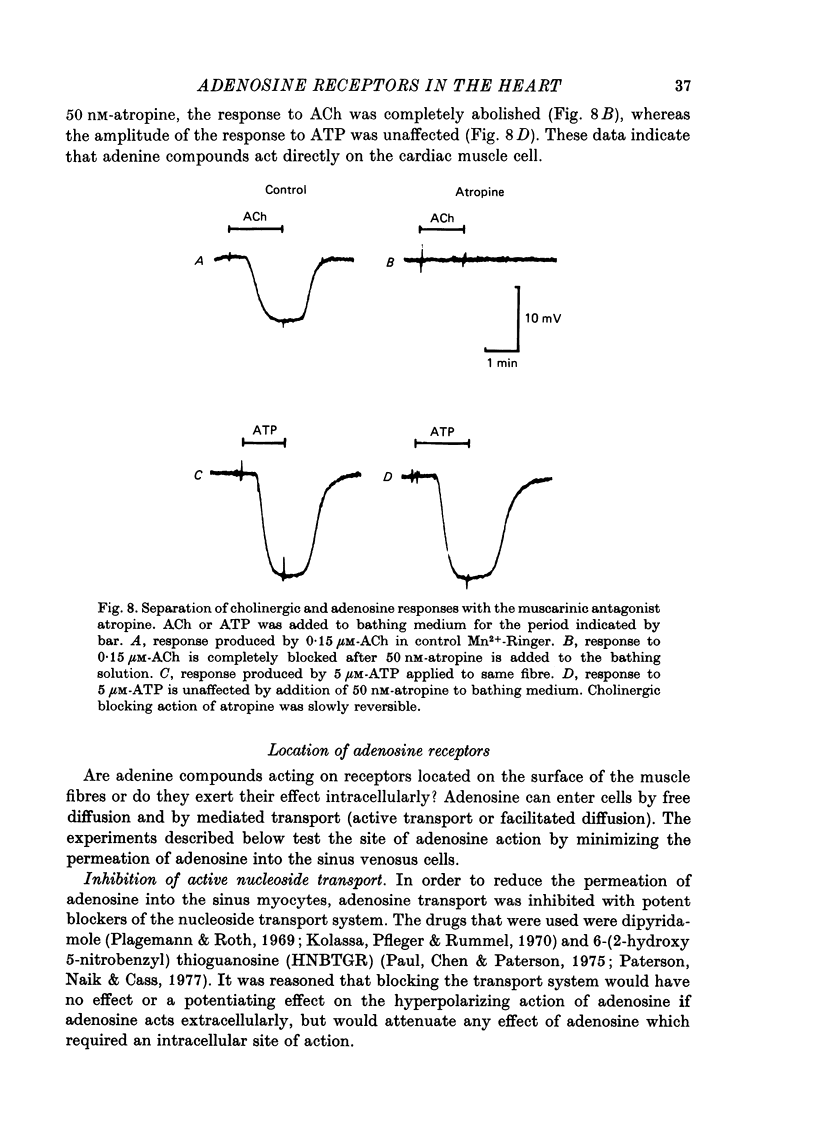
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Brooker G. Dissociation of cyclic GMP from the negative inotropic action of carbachol in guinea pig atria. J Cyclic Nucleotide Res. 1977 Dec;3(6):407–413. [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Clark R. B., Gross R., Su Y. F., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content in human astrocytoma cells by adenosine and the adenine nucleotides. J Biol Chem. 1974 Aug 25;249(16):5296–5303. [PubMed] [Google Scholar]
- DEGUBAREFF T., SLEATOR W., Jr EFFECTS OF CAFFEINE ON MAMMALIAN ATRIAL MUSCLE, AND ITS INTERACTION WITH ADENOSINE AND CALCIUM. J Pharmacol Exp Ther. 1965 May;148:202–214. [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
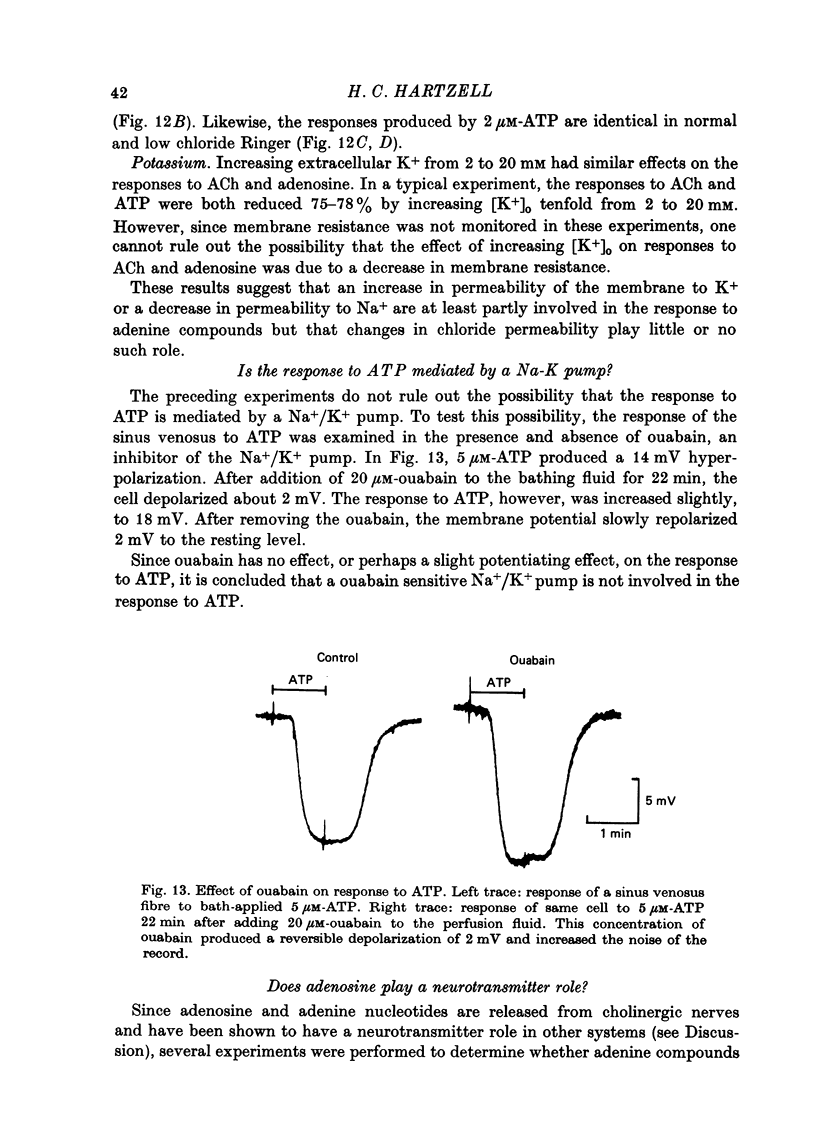
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
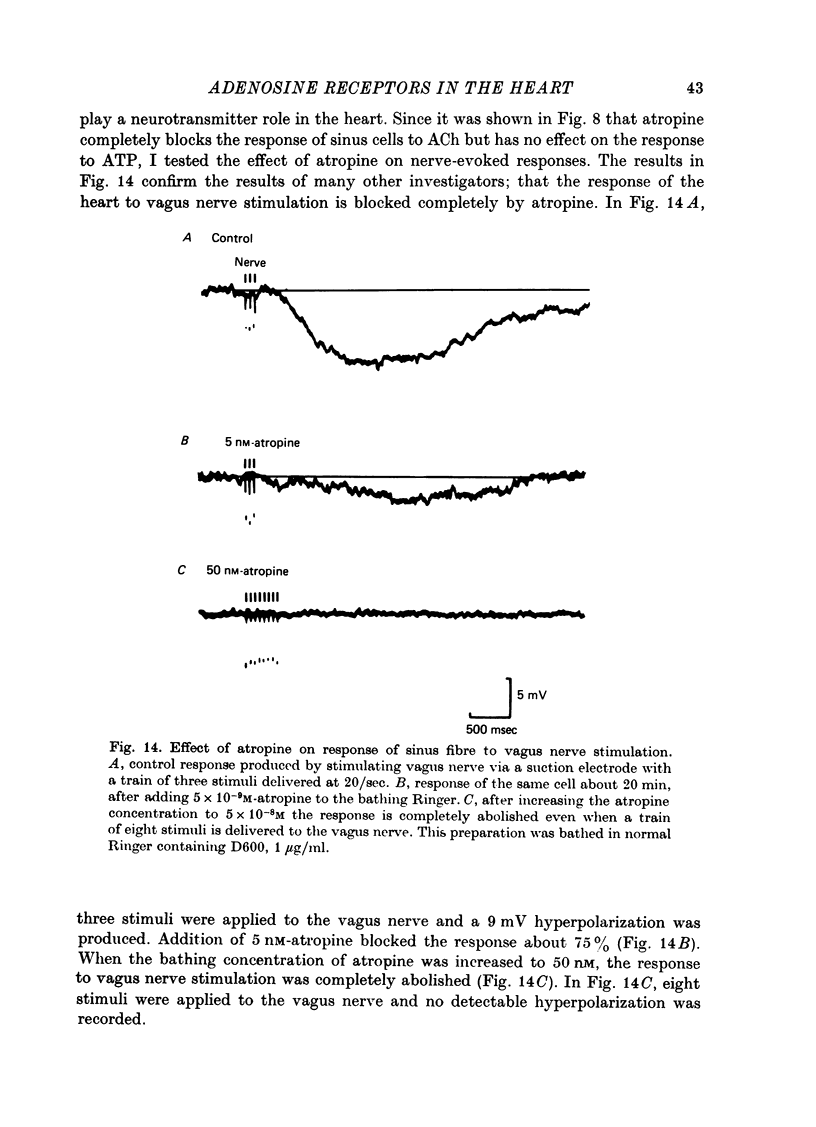
- Foley D. H., Herlihy J. T., Thompson C. I., Rubio R., Berne R. M. Increased adenosine formation by rat myocardium with acute aortic constriction. J Mol Cell Cardiol. 1978 Mar;10(3):293–300. doi: 10.1016/0022-2828(78)90352-8. [DOI] [PubMed] [Google Scholar]
- Garnier D., Nargeot J., Ojeda C., Rougier O. The action of acetylcholine on background conductance in frog atrial trabeculae. J Physiol. 1978 Jan;274:381–396. doi: 10.1113/jphysiol.1978.sp012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Pott L. Effects of acetylcholine and parasympathetic nerve stimulation on membrane potential in quiescent guinea-pig atria. J Physiol. 1978 Jun;279:655–668. doi: 10.1113/jphysiol.1978.sp012367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Yatani A., Tsuda Y. An analysis of the action of ATP and related compounds on membrane current and tension components in bullfrog atrial muscle. Jpn J Physiol. 1977;27(1):81–94. doi: 10.2170/jjphysiol.27.81. [DOI] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- HOLLANDER P. B., WEBB J. L. Effects of adenine nucleotides on the contractility and membrane potentials of rat atrium. Circ Res. 1957 Jul;5(4):349–353. doi: 10.1161/01.res.5.4.349. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Smith I., Purves R. D. Synaptic delay in the heart: an ionophoretic study. J Physiol. 1978 Jun;279:31–54. doi: 10.1113/jphysiol.1978.sp012329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S. V. The potentiation of the action of adenosine on the guinea-pig heart. Biochem Pharmacol. 1973 Feb 1;22(3):341–348. doi: 10.1016/0006-2952(73)90415-2. [DOI] [PubMed] [Google Scholar]
- Huang M., Drummond G. I. Effect of adenosine on cyclic AMP accumulation in ventricular myocardium. Biochem Pharmacol. 1976 Dec 15;25(24):2713–2719. doi: 10.1016/0006-2952(76)90262-8. [DOI] [PubMed] [Google Scholar]
- JAMES T. N. Arrhythmias and conduction disturbances in acute myocardial infarction. Am Heart J. 1962 Sep;64:416–426. doi: 10.1016/0002-8703(62)90158-8. [DOI] [PubMed] [Google Scholar]
- JOHNSON E. A., MCKINNON M. G. Effect of acetylcholine and adenosine on cardiac cellular potentials. Nature. 1956 Nov 24;178(4543):1174–1175. doi: 10.1038/1781174a0. [DOI] [PubMed] [Google Scholar]
- James T. N. The chronotropic action of ATP and related compounds studied by direct perfusion of the sinus node. J Pharmacol Exp Ther. 1965 Aug;149(2):233–247. [PubMed] [Google Scholar]
- Kolassa N., Pfleger K., Rummel W. Specificity of adenosine uptake into the heart and inhibition by dipyridamole. Eur J Pharmacol. 1970 Mar;9(3):265–268. doi: 10.1016/0014-2999(70)90221-9. [DOI] [PubMed] [Google Scholar]
- Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C. C., Hert R. C., Fain J. N. Characterization of [3H]adenosine binding to fat cell membranes. J Biol Chem. 1978 May 10;253(9):3114–3122. [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- Melnick R. L., De Sousa J. T., Magiure J., Packer L. Action of the adenosine triphosphate analog, adenylyl imidodiphosphate in mitochondria. Arch Biochem Biophys. 1975 Jan;166(1):139–144. doi: 10.1016/0003-9861(75)90373-2. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Atkinson M. R. Adenosine 5'-phosphorothioate. A nucleotide analog that is a substrate, competitive inhibitor, or regulator of some enzymes that interact with adenosine 5'-phosphate. Biochemistry. 1968 Nov;7(11):4023–4029. doi: 10.1021/bi00851a032. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Davis C. J., Khouri E. M., Patterson R. E. Evidence for an adenosine receptor on the surface of dog coronary myocytes. Circ Res. 1976 Jul;39(1):93–98. doi: 10.1161/01.res.39.1.93. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Kim S. C., Bernard O., Cass C. E. Transport of nucleosides. Ann N Y Acad Sci. 1975 Aug 8;255:402–411. doi: 10.1111/j.1749-6632.1975.tb29248.x. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Naik S. R., Cass C. E. Inhibition of uridine uptake in HeLa cells by nitrobenzylthioinosine and related compounds. Mol Pharmacol. 1977 Nov;13(6):1014–1023. [PubMed] [Google Scholar]
- Paul B., Chen M. F., Paterson A. R. Inhibitors of nucleoside transport. A structure-activity study using human erythrocytes. J Med Chem. 1975 Oct;18(10):968–973. doi: 10.1021/jm00244a003. [DOI] [PubMed] [Google Scholar]
- Pfleger K., Volkmer I., Kolassa N. Hemmung der Aufnahme von Adenosin und Verstärkung seiner Wirkung am isolierten Warmblüterherzen durch coronarwirksame Substanzen. Arzneimittelforschung. 1969 Dec;19(12):1972–1974. [PubMed] [Google Scholar]
- Pfleger K., Volkmer I., Kolassa N. Hemmung der Aufnahme von Adenosin und Verstärkung seiner Wirkung am isolierten Warmblüterherzen durch coronarwirksame Substanzen. Arzneimittelforschung. 1969 Dec;19(12):1972–1974. [PubMed] [Google Scholar]
- Plagemann P. G., Roth M. F. Permeation as the rate-limiting step in the phosphorylation of uridine and choline and their incorporation into macromolecules by Novikoff hepatoma cells. Competitive inhibition by phenethyl alcohol, persantin, and adenosine. Biochemistry. 1969 Dec;8(12):4782–4789. doi: 10.1021/bi00840a020. [DOI] [PubMed] [Google Scholar]
- Purves R. D. Function of muscarinic and nicotinic acetylcholine receptors. Nature. 1976 May 13;261(5556):149–151. doi: 10.1038/261149a0. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A. ATP; related nucleotides and adenosine on neurotransmission. Life Sci. 1978 Apr 24;22(16):1373–1380. doi: 10.1016/0024-3205(78)90630-6. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Oye I., Morgan H. E., Sutherland E. W. The effect of epinephrine on adenosine 3', 5'-phosphate levels in the isolated perfused rat heart. Mol Pharmacol. 1965 Sep;1(2):168–177. [PubMed] [Google Scholar]
- Schrader J., Gerlach E. Compartmentation of cardiac adenine nucleotides and formation of adenosine. Pflugers Arch. 1976 Dec 28;367(2):129–135. doi: 10.1007/BF00585148. [DOI] [PubMed] [Google Scholar]
- Schrader J., Haddy F. J., Gerlach E. Release of adenosine, inosine and hypoxanthine from the isolated guinea pig heart during hypoxia, flow-autoregulation and reactive hyperemia. Pflugers Arch. 1977 May 6;369(1):1–6. doi: 10.1007/BF00580802. [DOI] [PubMed] [Google Scholar]
- Schrader J., Nees S., Gerlach E. Evidence for a cell surface adenosine receptor on coronary myocytes and atrial muscle cells. Studies with an adenosine derivative of high molecular weight. Pflugers Arch. 1977 Jul 19;369(3):251–257. doi: 10.1007/BF00582192. [DOI] [PubMed] [Google Scholar]
- Schrader J., Rubio R., Berne R. M. Inhibition of slow action potentials of guinea pig atrial muscle by adenosine: a possible effect on Ca2+ influx. J Mol Cell Cardiol. 1975 Jun;7(6):427–433. doi: 10.1016/0022-2828(75)90048-6. [DOI] [PubMed] [Google Scholar]
- Stafford A. Potentiation of adenosine and the adenine nucleotides by dipyridamole. Br J Pharmacol Chemother. 1966 Nov;28(2):218–227. doi: 10.1111/j.1476-5381.1966.tb01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Eick R., Nawrath H., McDonald T. F., Trautwein W. On the mechanism of the negative inotropic effect of acetylcholine. Pflugers Arch. 1976 Feb 24;361(3):207–213. doi: 10.1007/BF00587284. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Walker J. L., Ladle R. O. Frog heart intracellular potassium activities measured with potassium microelectrodes. Am J Physiol. 1973 Jul;225(1):263–267. doi: 10.1152/ajplegacy.1973.225.1.263. [DOI] [PubMed] [Google Scholar]
- Wiedmeier V. T., Rubio R., Berne R. M. Incorporation and turnover of adenosine-U- 14 C in perfused guinea pig myocardium. Am J Physiol. 1972 Jul;223(1):51–54. doi: 10.1152/ajplegacy.1972.223.1.51. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine inhibits the accumulation of cyclic AMP in cultured brain cells. Nature. 1978 Dec 21;276(5690):839–841. doi: 10.1038/276839a0. [DOI] [PubMed] [Google Scholar]


