Abstract
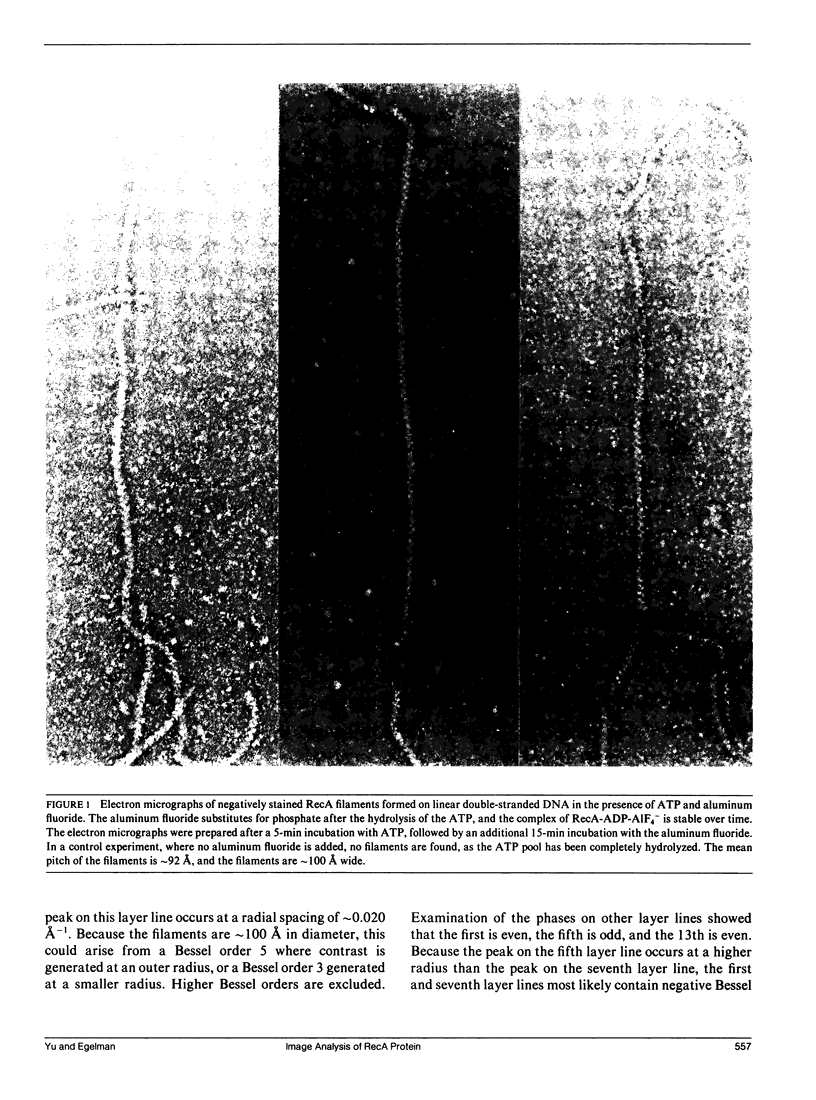
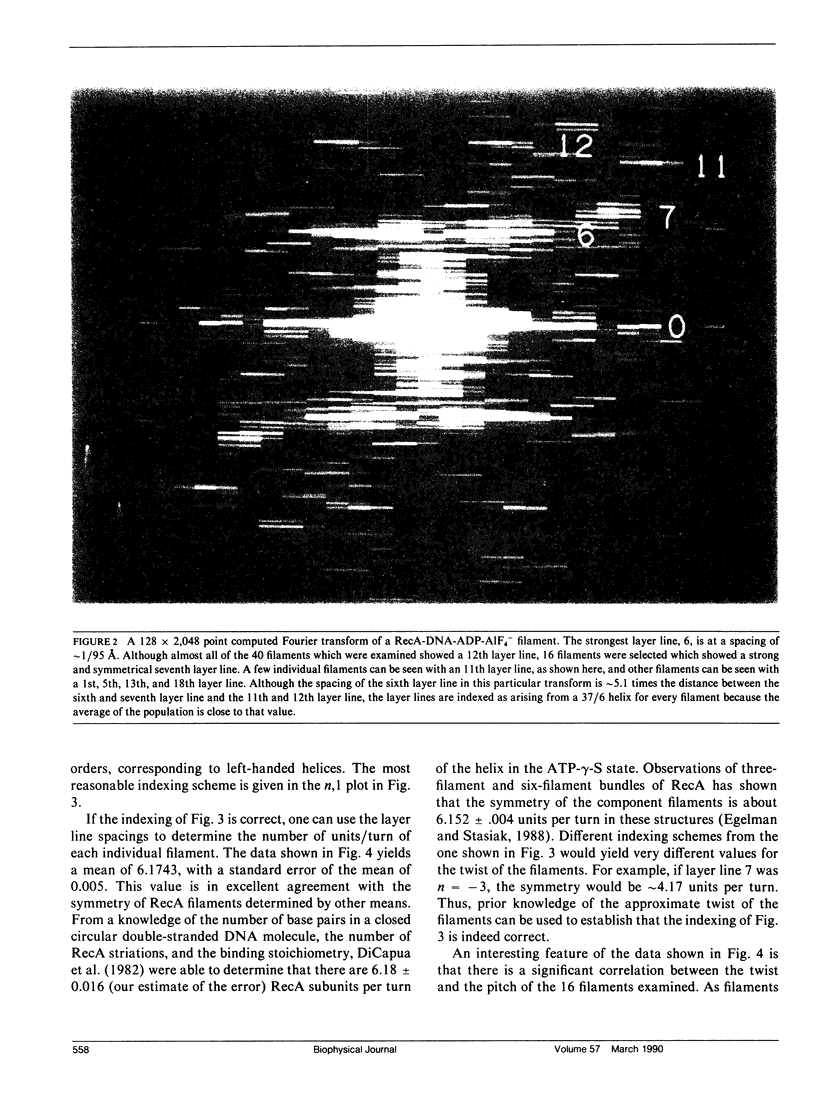
The Escherichia coli RecA protein catalyzes homologous genetic recombination by forming helical polymers around DNA molecules. These polymers have an ATPase activity, which is essential for the movement of strands between two DNA molecules. One obstacle to structural studies of the RecA filament has been that the ATPase results in a dynamical polymer containing a mixture of states with respect to the bound ATP and its hydrolytic products. We have formed filaments which are trapped in the ADP-Pi state by substituting AIF4- for the Pi, and have used these stable filaments to generate a three-dimensional reconstruction from electron micrographs. The resolution of the reconstruction is sufficient to resolve the 38-k RecA subunit into two nearly equal domains. This reconstruction provides the most detailed view yet of the RecA protein, and serves as a framework within which existing biochemical data on RecA can be understood.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner S. L., Mitchell R. S., Morrical S. W., Neuendorf S. K., Schutte B. C., Cox M. M. recA protein-promoted ATP hydrolysis occurs throughout recA nucleoprotein filaments. J Biol Chem. 1987 Mar 25;262(9):4011–4016. [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Di Capua E., Engel A., Stasiak A., Koller T. Characterization of complexes between recA protein and duplex DNA by electron microscopy. J Mol Biol. 1982 May 5;157(1):87–103. doi: 10.1016/0022-2836(82)90514-9. [DOI] [PubMed] [Google Scholar]
- Egelman E. H. An algorithm for straightening images of curved filamentous structures. Ultramicroscopy. 1986;19(4):367–373. doi: 10.1016/0304-3991(86)90096-3. [DOI] [PubMed] [Google Scholar]
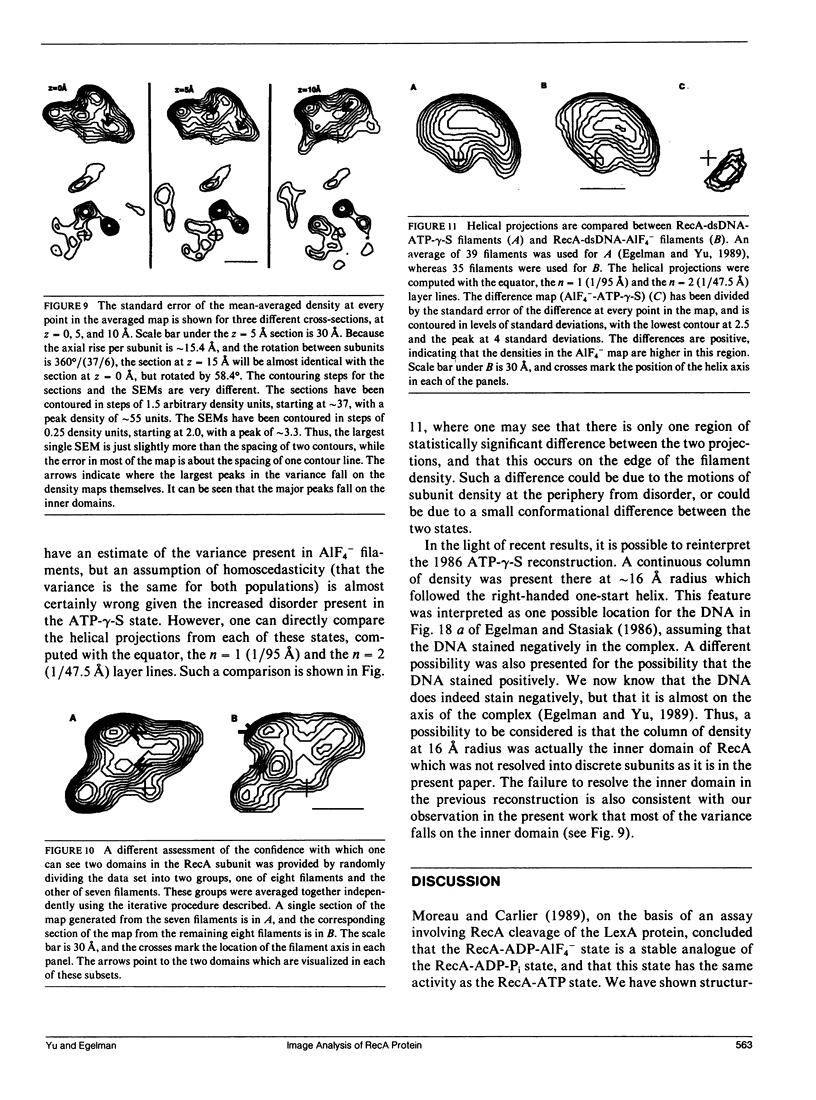
- Egelman E. H., Stasiak A. Structure of helical RecA-DNA complexes. Complexes formed in the presence of ATP-gamma-S or ATP. J Mol Biol. 1986 Oct 20;191(4):677–697. doi: 10.1016/0022-2836(86)90453-5. [DOI] [PubMed] [Google Scholar]
- Egelman E. H., Stasiak A. Structure of helical RecA-DNA complexes. II. Local conformational changes visualized in bundles of RecA-ATP gamma S filaments. J Mol Biol. 1988 Mar 20;200(2):329–349. doi: 10.1016/0022-2836(88)90245-8. [DOI] [PubMed] [Google Scholar]
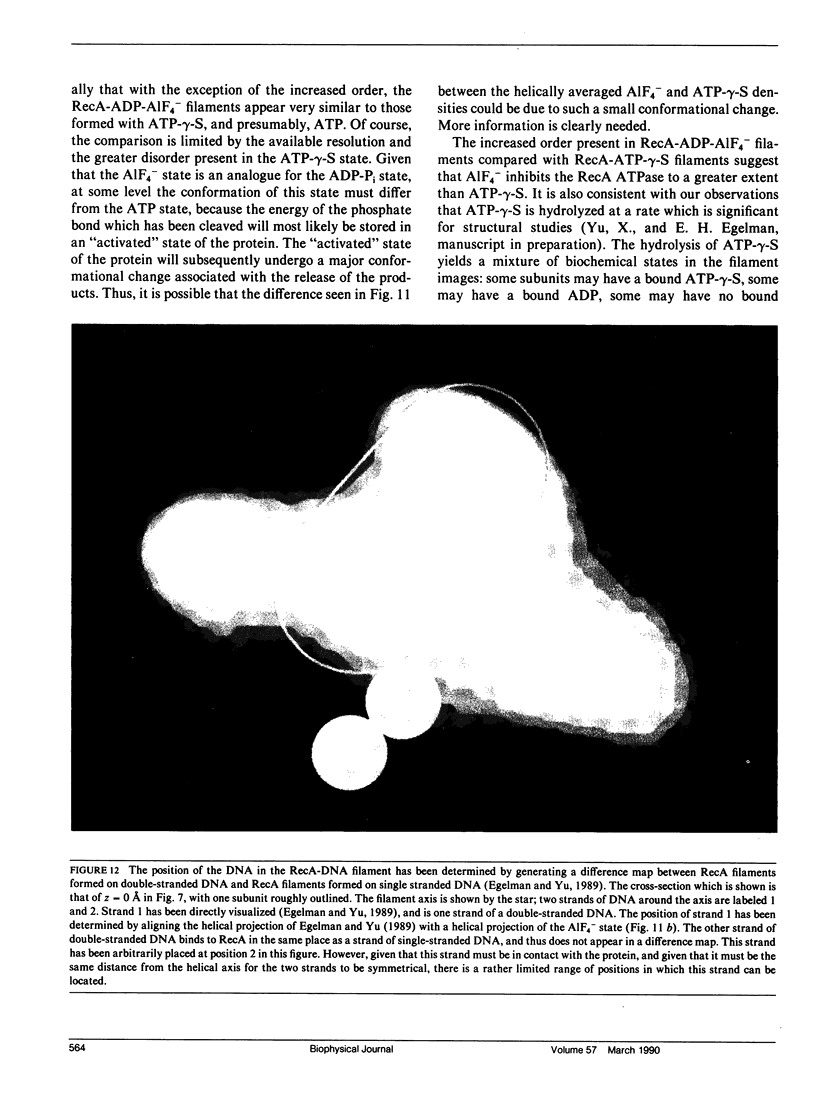
- Egelman E. H., Yu X. The location of DNA in RecA-DNA helical filaments. Science. 1989 Jul 28;245(4916):404–407. doi: 10.1126/science.2667137. [DOI] [PubMed] [Google Scholar]
- Flory J., Tsang S. S., Muniyappa K. Isolation and visualization of active presynaptic filaments of recA protein and single-stranded DNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7026–7030. doi: 10.1073/pnas.81.22.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J., Shores C. G. RecA protein rapidly crystallizes in the presence of spermidine: a valuable step in its purification and physical characterization. Biochemistry. 1985 Jan 1;24(1):158–162. doi: 10.1021/bi00322a022. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., West S. C., Stasiak A. Role of RecA protein spiral filaments in genetic recombination. Nature. 1984 May 17;309(5965):215–219. doi: 10.1038/309215a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Knight K., McEntee K. Evidence for nucleotide-mediated changes in the domain structure of the recA protein of Escherichia coli. Biochemistry. 1987 Oct 20;26(21):6801–6810. doi: 10.1021/bi00395a033. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Moreau P. L., Carlier M. F. RecA protein-promoted cleavage of LexA repressor in the presence of ADP and structural analogues of inorganic phosphate, the fluoride complexes of aluminum and beryllium. J Biol Chem. 1989 Feb 5;264(4):2302–2306. [PubMed] [Google Scholar]
- Phizicky E. M., Roberts J. W. Induction of SOS functions: regulation of proteolytic activity of E. coli RecA protein by interaction with DNA and nucleoside triphosphate. Cell. 1981 Jul;25(1):259–267. doi: 10.1016/0092-8674(81)90251-8. [DOI] [PubMed] [Google Scholar]
- Stasiak A., Di Capua E. The helicity of DNA in complexes with recA protein. Nature. 1982 Sep 9;299(5879):185–186. doi: 10.1038/299185a0. [DOI] [PubMed] [Google Scholar]
- Stasiak A., Egelman E. H. Structure and Dynamics of recA Protein-DNA Complexes as Determined by Image Analysis of Electron Micrographs. Biophys J. 1986 Jan;49(1):5–7. doi: 10.1016/S0006-3495(86)83569-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak A., Stasiak A. Z., Koller T. Visualization of RecA-DNA complexes involved in consecutive stages of an in vitro strand exchange reaction. Cold Spring Harb Symp Quant Biol. 1984;49:561–570. doi: 10.1101/sqb.1984.049.01.063. [DOI] [PubMed] [Google Scholar]
- Suck D., Kabsch W., Mannherz H. G. Three-dimensional structure of the complex of skeletal muscle actin and bovine pancreatic DNAse I at 6-A resolution. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4319–4323. doi: 10.1073/pnas.78.7.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- West S. C., Cassuto E., Howard-Flanders P. Postreplication repair in E. coli: strand exchange reactions of gapped DNA by RecA protein. Mol Gen Genet. 1982;187(2):209–217. doi: 10.1007/BF00331119. [DOI] [PubMed] [Google Scholar]