Abstract
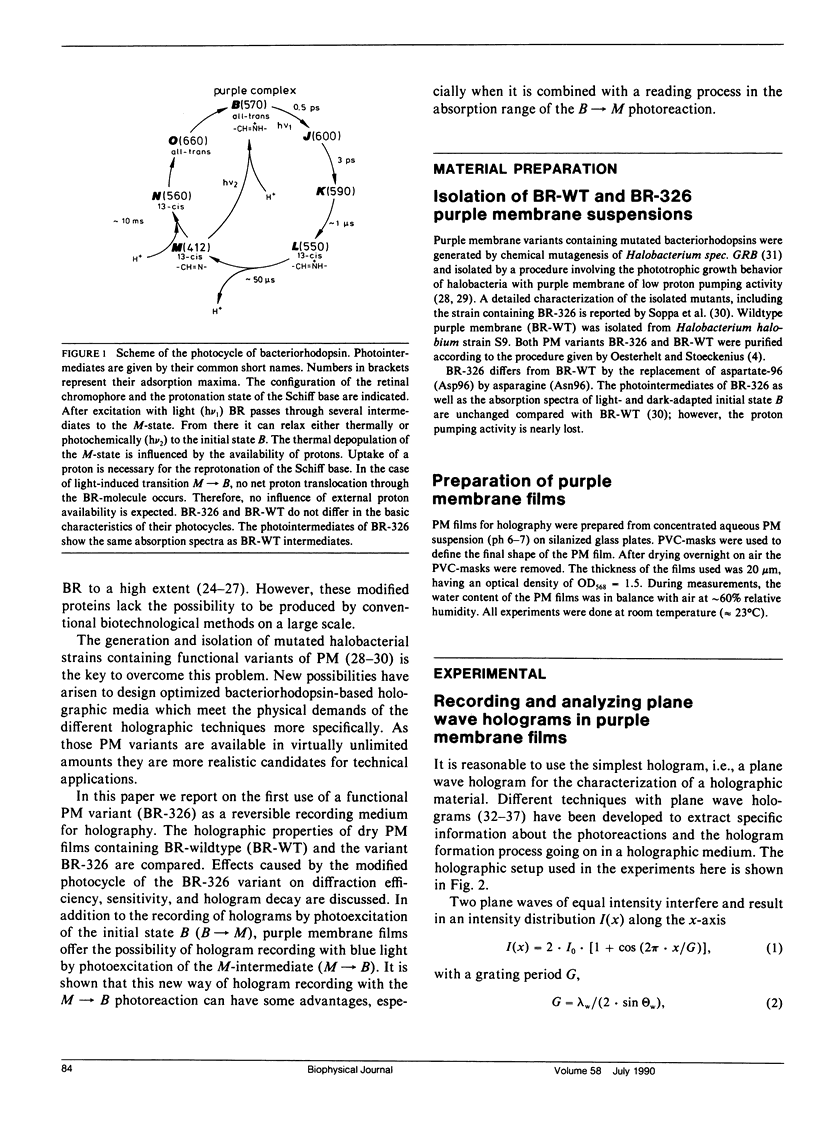
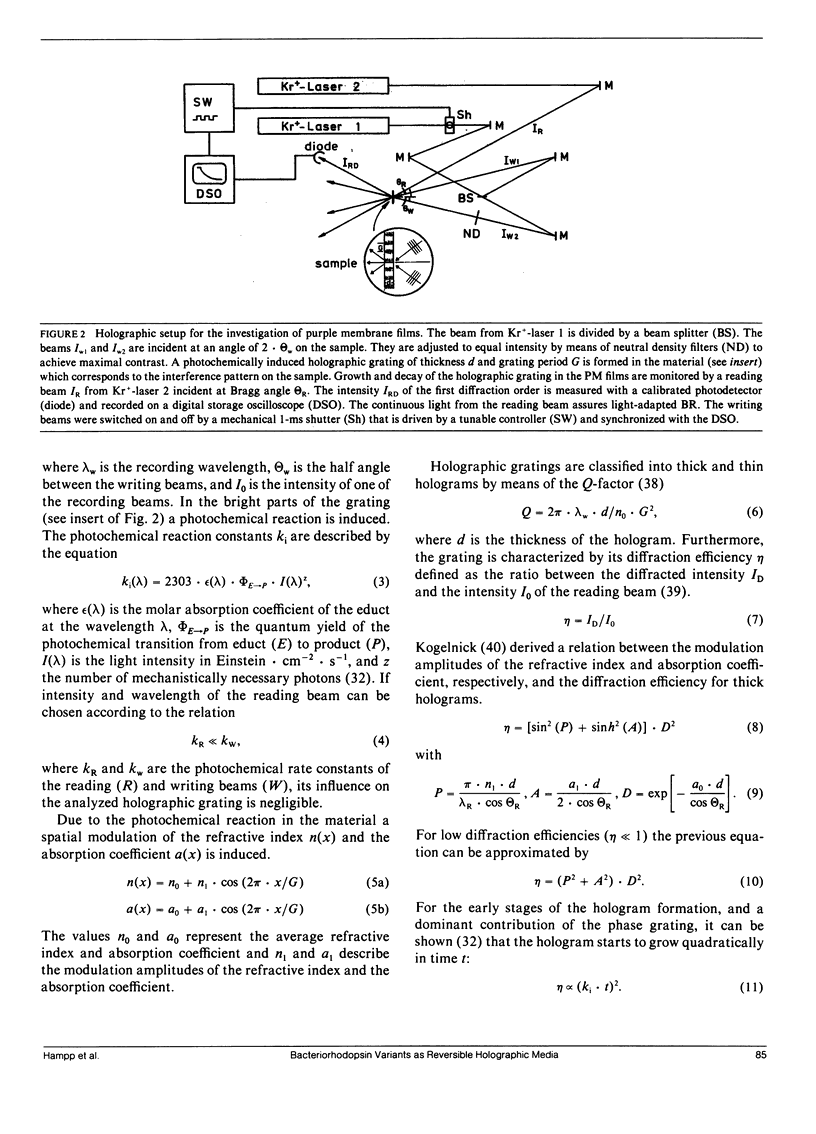
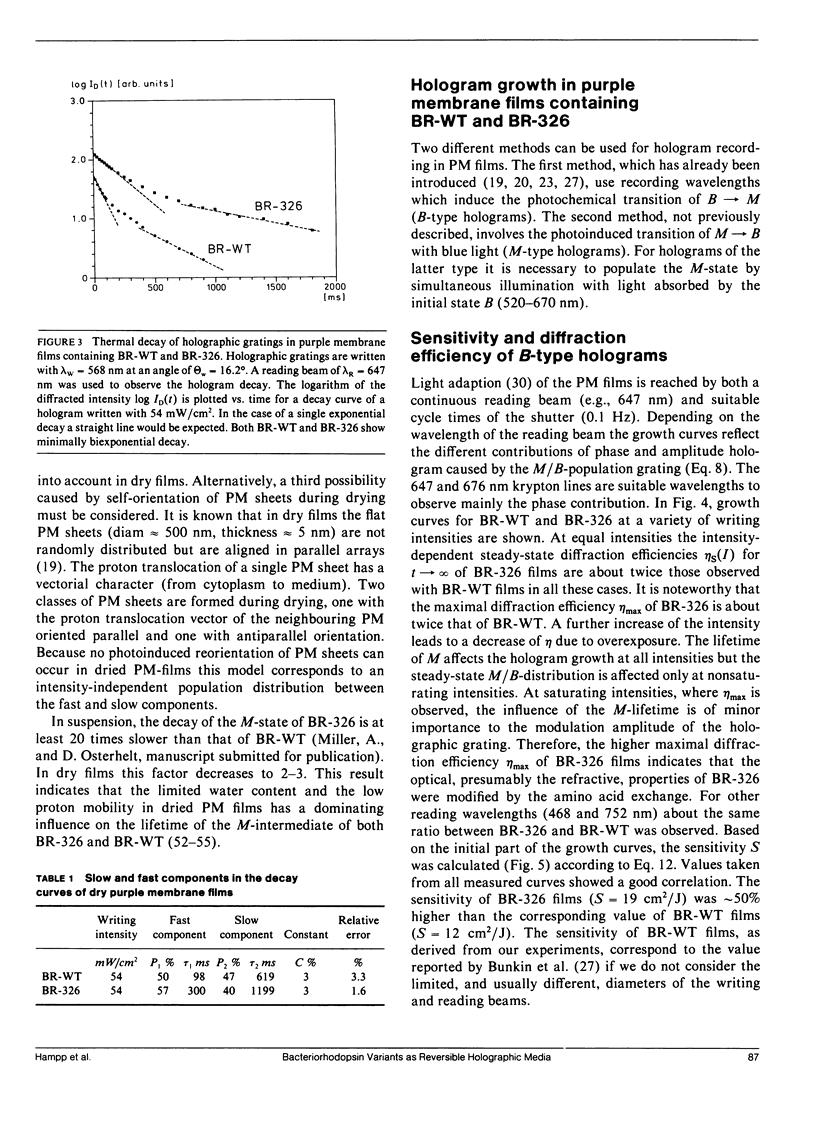
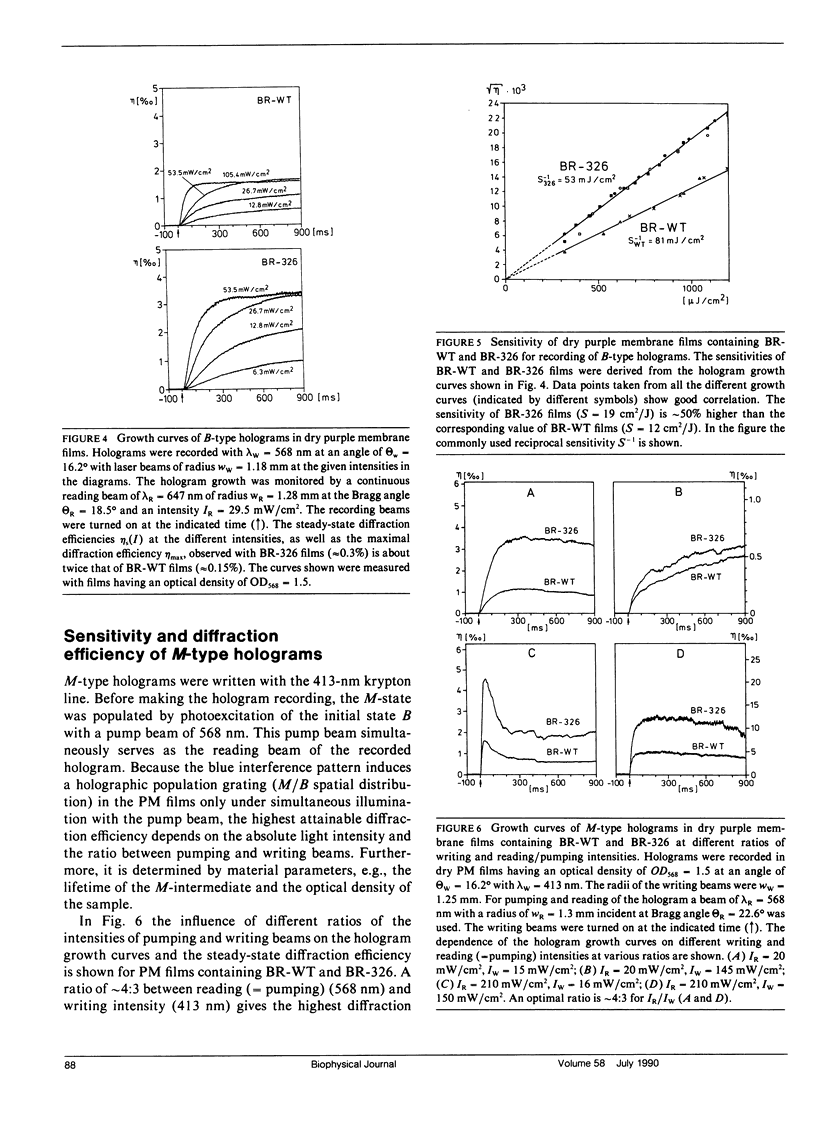
Air dried films of purple membranes (PM) from Halobacterium halobium containing the photochromic protein bacteriorhodopsin (BR) were prepared and the BR-photocycle of this material analyzed. The absorption maxima of the initial state B (λmax = 570 nm) and the photochemical intermediate M (λmax = 412 nm), which is the longest living intermediate in suspension (τ ≈ 10 ms), were spectrally well separated. Light-induced population gratings between B and M were used for reversible holographic recording in these dry PM films. The resolution (>5,000 lines/mm) of PM films was comparable to the corresponding values of conventional photochromic recording materials. The longterm stability toward photochemical degradation of PM films is excellent (> 100.000 recording cycles). The spectral bandwidth (400-680 nm) of such films covers nearly the whole visible spectrum. Both the photochemical transition from B → M with wavelengths in the green-red range and from M → B with blue light were utilized for holographic recording. The latter possibility (M → B) seems to be advantageous for several applications because the holographic grating is only formed during reconstruction. Higher reading intensities lead to higher population of the M-state and result in an increase of the fringe contrast instead of decreasing it. New possibilities for the further development of holographic media based on bacteriorhodopsin are raised by the availability of PM variants with modified optical properties. By the use of the variant BR-326, which differs from the wildtype PM by a single amino acid exchange (aspartate-96 → asparagine), the sensitivity of PM films is increased by ∼50% from 12 cm2/J to 19 cm2/J for recording with 568 nm. The sensitivity for recording with 413 nm (33 cm2/J) is not influenced by the amino acid exchange. The observed diffraction efficiency η of PM films with BR-326 is twice that of BR-wildtype (BR-WT) films and is in the range of conventional organic photochromics (≈ 1%). In dried films of both BR-WT and BR-326 the M-decay was shown to be at least biexponential.
Full text
PDF




Selected References
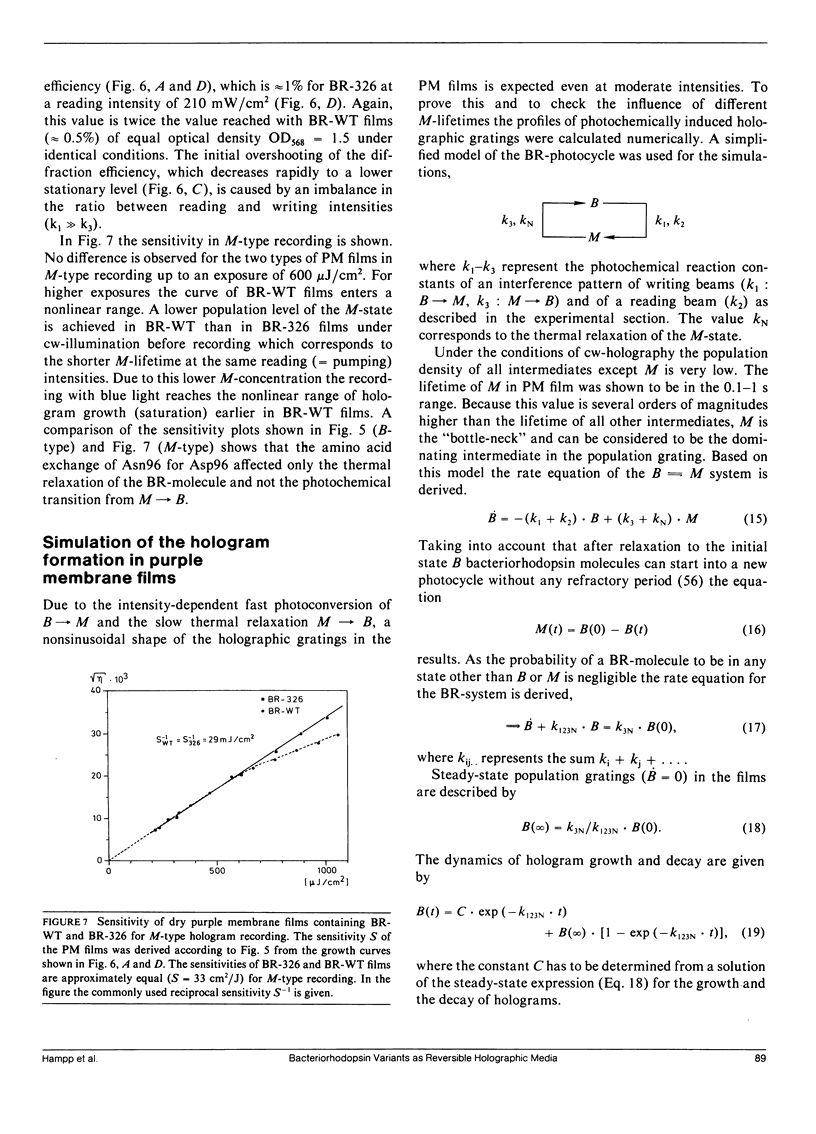
These references are in PubMed. This may not be the complete list of references from this article.
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Dunn R. J., Hackett N. R., McCoy J. M., Chao B. H., Kimura K., Khorana H. G. Structure-function studies on bacteriorhodopsin. I. Expression of the bacterio-opsin gene in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9246–9254. [PubMed] [Google Scholar]
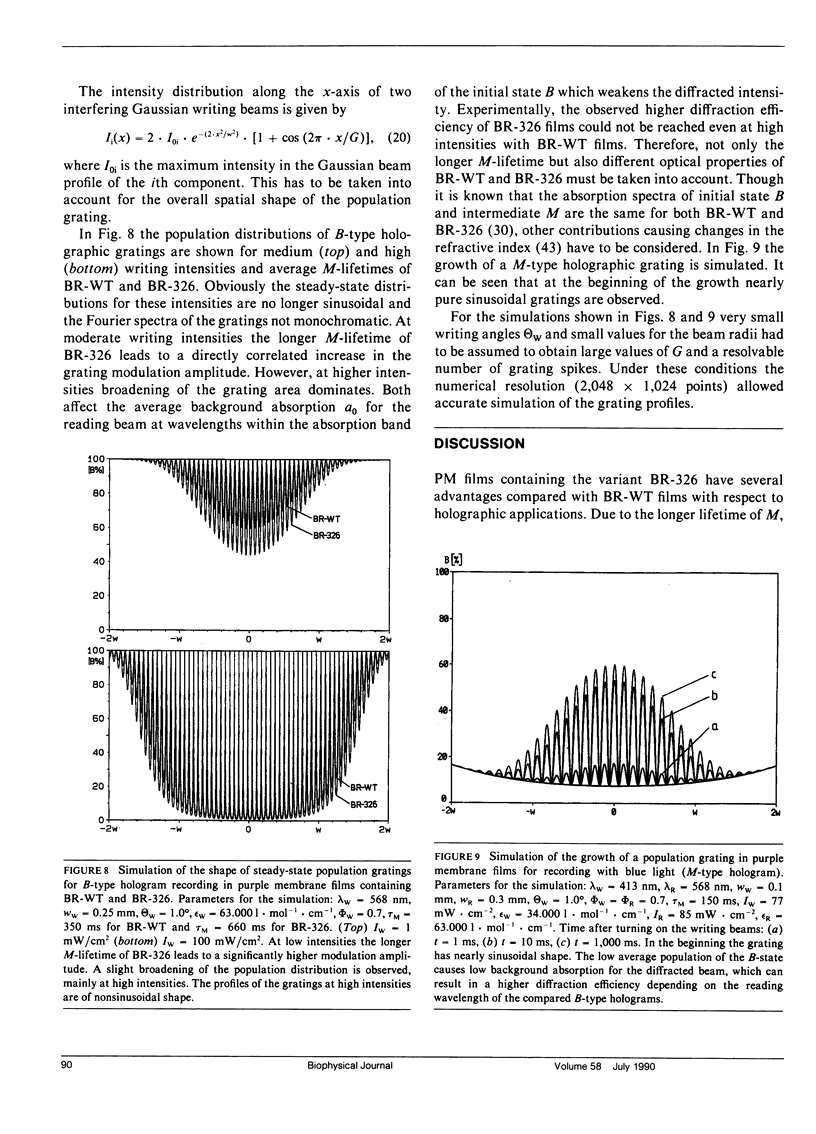
- Dér A., Hargittai P., Simon J. Time-resolved photoelectric and absorption signals from oriented purple membranes immobilized in gel. J Biochem Biophys Methods. 1985 Mar;10(5-6):295–300. doi: 10.1016/0165-022x(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Eisenbach M., Weissmann C., Tanny G., Caplan S. R. Bacteriorhodopsin-loaded charged synthetic membranes. Utilization of light energy to generate electrical current. FEBS Lett. 1977 Sep 1;81(1):77–80. doi: 10.1016/0014-5793(77)80932-0. [DOI] [PubMed] [Google Scholar]
- Groma G. I., Dancshazy Z. How Many M Forms are there in the Bacteriorhodopsin Photocycle? Biophys J. 1986 Aug;50(2):357–366. doi: 10.1016/S0006-3495(86)83469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groma G. I., Helgerson S. L., Wolber P. K., Beece D., Dancsházy Z., Keszthelyi L., Stoeckenius W. Coupling between the bacteriorhodopsin photocycle and the protonmotive force in Halobacterium halobium cell envelope vesicles. II. Quantitation and preliminary modeling of the M----bR reactions. Biophys J. 1984 May;45(5):985–992. doi: 10.1016/S0006-3495(84)84243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner W., Oesterhelt D. Methoxyretinals in bacteriorhodopsin. Absorption maxima, cis-trans isomerization and retinal protein interaction. Eur J Biochem. 1988 Oct 1;176(3):641–648. doi: 10.1111/j.1432-1033.1988.tb14325.x. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Holz M., Drachev L. A., Mogi T., Otto H., Kaulen A. D., Heyn M. P., Skulachev V. P., Khorana H. G. Replacement of aspartic acid-96 by asparagine in bacteriorhodopsin slows both the decay of the M intermediate and the associated proton movement. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2167–2171. doi: 10.1073/pnas.86.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F. T. The bacteriorhodopsin model membrane system as a prototype molecular computing element. Biosystems. 1986;19(3):223–236. doi: 10.1016/0303-2647(86)90041-9. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on the photocycle of bacteriorhodopsin in thin layers of purple membrane. Nature. 1977 Nov 10;270(5633):184–186. doi: 10.1038/270184a0. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Kinosita K., Jr, Ikegami A. Structure and function of bacteriorhodopsin. Adv Biophys. 1988;24:123–175. doi: 10.1016/0065-227x(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Lazarev Y. A., Terpugov E. L. Effect of water on the structure of bacteriorhodopsin and photochemical processes in purple membranes. Biochim Biophys Acta. 1980 May 9;590(3):324–338. doi: 10.1016/0005-2728(80)90203-0. [DOI] [PubMed] [Google Scholar]
- Lemke H. D., Oesterhelt D. Lysine 216 is a binding site of the retinyl moiety in bacteriorhodopsin. FEBS Lett. 1981 Jun 15;128(2):255–260. doi: 10.1016/0014-5793(81)80093-2. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D. Bacteriorhodopsin as a light-driven ion exchanger? FEBS Lett. 1976 Apr 15;64(1):20–22. doi: 10.1016/0014-5793(76)80238-4. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Krippahl G. Phototrophic growth of halobacteria and its use for isolation of photosynthetically-deficient mutants. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):137–150. doi: 10.1016/s0769-2609(83)80101-x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Soppa J., Oesterhelt D. Bacteriorhodopsin mutants of Halobacterium sp. GRB. I. The 5-bromo-2'-deoxyuridine selection as a method to isolate point mutants in halobacteria. J Biol Chem. 1989 Aug 5;264(22):13043–13048. [PubMed] [Google Scholar]
- Soppa J., Otomo J., Straub J., Tittor J., Meessen S., Oesterhelt D. Bacteriorhodopsin mutants of Halobacterium sp. GRB. II. Characterization of mutants. J Biol Chem. 1989 Aug 5;264(22):13049–13056. [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Keszthelyi L. Photoelectric signals from dried oriented purple membranes of Halobacterium halobium. Biophys J. 1983 Jul;43(1):47–51. doi: 10.1016/S0006-3495(83)84322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


