Abstract
Neisseria gonorrhoeae and Neisseria meningitidis express an ∼43-kDa α-2,3-sialyltransferase (Lst) that sialylates the surface lipooligosaccharide (LOS) by using exogenous (in all N. gonorrhoeae strains and some N. meningitidis serogroups) or endogenous (in other N. meningitidis serogroups) sources of 5′-cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA). Sialylation of LOS can protect N. gonorrhoeae and N. meningitidis from complement-mediated serum killing and from phagocytic killing by neutrophils. The precise subcellular location of Lst has not been determined. We confirm and extend previous studies by demonstrating that Lst is located in the outer membrane and is surface exposed in both N. gonorrhoeae and N. meningitidis. Western immunoblot analysis of subcellular fractions of N. gonorrhoeae strain F62 and N. meningitidis strain MC58⊄3 (an acapsulate serogroup B strain) performed with rabbit antiserum raised against recombinant Lst revealed an ∼43-kDa protein exclusively in outer membrane preparations of both pathogens. Inner membrane, periplasmic, cytoplasmic, and culture supernatant fractions were devoid of Lst, as determined by Western blot analysis. Consistent with this finding, outer membrane fractions of N. gonorrhoeae were significantly enriched for sialyltransferase enzymatic activity. A trace of enzymatic activity was detected in inner membrane fractions, which may have represented Lst in transit to the outer membrane or may have represented inner membrane contamination of outer membrane preparations. Subcellular preparations of an isogenic lst insertion knockout mutant of N. gonorrhoeae F62 (strain ST01) expressed neither a 43-kDa immunoreactive protein nor sialyltransferase activity. Anti-Lst rabbit antiserum bound to whole cells of N. meningitidis MC58⊄3 and wild-type N. gonorrhoeae F62 but not to the Lst mutant ST01, indicating the surface exposure of the enzyme. Although the anti-Lst antiserum avidly bound enzymatically active, recombinant Lst, it inhibited Lst (sialyltransferase) activity by only about 50% at the highest concentration of antibody used. On the contrary, anti-Lst antiserum did not inhibit sialylation of whole N. gonorrhoeae cells in the presence of exogenous CMP-NANA, suggesting that the antibody did not bind to or could not access the enzyme active site on the surface of viable Neisseria cells. Taken together, these results indicate that Lst is an outer membrane, surface-exposed glycosyltransferase. To our knowledge, this is the first demonstration of the localization of a bacterial glycosyltransferase to the outer membrane of gram-negative bacteria.
The genus Neisseria consists of two major human pathogens, Neisseria gonorrhoeae and Neisseria meningitidis. N. gonorrhoeae is the etiological agent of the sexually transmitted disease gonorrhea, while N. meningitidis is a cause of bacterial sepsis and meningitis worldwide. Despite the contrast in the diseases caused by these two organisms, they share strategies to evade the human immune response during infection, including antigenic and phase variation and masking of immunogenic surface molecules (10, 25). These systems interfere with elicitation of protective immunity and present challenges to the development of vaccines against these organisms. In particular, serogroup B and C strains of N. meningitidis express polysaccharide capsules composed of homopolymers of sialic acid which prevent proper deposition of bactericidal components of the complement system (23). Gonococci are not encapsulated, but along with meningococci, they exhibit monosialylated lipooligosaccharide (LOS) which blocks complement-dependent killing through the binding of factor H (23). The extent to which LOS sialylation confers serum resistance upon a meningococcus, in lieu of its polysaccharide capsule, remains a matter of debate, and the extents may be different in different serogroups or at different times during meningococcal infection and disease (9, 30). However, LOS sialylation is required by serum-sensitive N. gonorrhoeae to evade serum killing in vitro, and N. gonorrhoeae strains in urethral exudates from infected males are sialylated, suggesting that there is a role for this modification of LOS in the pathogenesis of gonorrhea (12).
Sialylation of LOS is catalyzed by an α-2,3-sialyltransferase (Lst) that monosialylates the terminal galactose of LOS by using 5′-cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA) (3, 12, 27). CMP-NANA is obtained from the host by N. gonorrhoeae; CMP-NANA is not synthesized by N. gonorrhoeae. However, N. meningitidis serogroups B and C can utilize endogenously synthesized CMP-NANA. Lst has been cloned and expressed in Escherichia coli and has been found to be a monomeric, 42.6-kDa protein that exhibits approximately 95 to 98% identity in strains of N. meningitidis and N. gonorrhoeae (3). Lst does not share sequence homology with eukaryotic sialyltransferases and exhibits broader acceptor specificity, having the ability to sialylate alpha- and beta-linked terminal galactose residues (3). Sialyltransferase activity is recovered almost exclusively in pellets of broken cell preparations of N. meningitidis and recombinant E. coli upon ultracentrifugation, indicating a membrane association (3). Outer membrane localization of Lst has been suggested by the ease with which Lst activity is extracted with Triton X-100 and by the dependence of N. gonorrhoeae on an external source of CMP-NANA (2). The demonstration of a noncleavable signal sequence at the N terminus of Lst further supports membrane localization, along with the known inner membrane distribution of glycosyltransferases involved in Salmonella lipopolysaccharide (LPS) biosynthesis (19). Although it has not been proven experimentally, it is reasonable to assume that the LOS biosynthetic enzymes of Neisseria have a distribution similar to that of the LPS biosynthetic enzymes of the Enterobacteriaceae (5). These observations support membrane association but do not unequivocally assign Lst to an inner or outer membrane location. Intact wild-type gonococci absorb radiolabeled CMP-NANA under cold conditions, suggesting that Lst is surface associated (2). However, this does not eliminate the possibility of an inner membrane location for Lst if a CMP-NANA transport system exists. The possibility of an inner membrane-associated Lst is supported by the ability of some N. meningitidis serogroups to endogenously sialylate LOS. Another characteristic of Lst is its constitutive expression, although various growth conditions result in modest increases or decreases in Lst activity in whole cells of N. gonorrhoeae, by as-yet-unknown mechanisms (1, 16, 22). If Lst is surface exposed, it would be one of a limited number of antigenically invariable proteins constitutively expressed on the surface of pathogenic neisseriae. Thus, because of its antigenic stability and its constitutive expression, Lst may be a vaccine candidate.
N. gonorrhoeae F62 and N. meningitidis MC58⊄3 express Lst molecules that share ∼95% identity at the amino acid level. In this study we demonstrated an outer membrane distribution and surface exposure of Lst by using subcellular fractions of N. gonorrhoeae F62 and N. meningitidis MC58⊄3 and anti-Lst antiserum. To our knowledge, Lst is the first glycosyltransferase localized to the outer membrane of gram-negative bacteria.
MATERIALS AND METHODS
Bacterial strains.
Stocks of N. gonorrhoeae strains F62 and ST01 that were predominately Opa negative and nonpiliated and N. meningitidis strain MC58⊄ 3 (an acapsulate serogroup B mutant) were used for all studies (17, 29). Strain ST01 is an lst knockout mutant of strain F62, created by transformation of F62 with an lst construct from N. meningitidis MC58⊄3 that has a kanamycin resistance cassette in lst. ST01 is serum sensitive in the presence of CMP-NANA (25 μg/ml) and completely lacks sialyltransferase enzymatic activity (data not shown).
Generation of Lst antisera.
Two different antisera were raised against two different recombinant forms of Lst. A recombinant Lst from N. meningitidis MC58⊄3, fused with c-Myc (designated cLst), was a gift from the laboratory of Warren Wakarchuk at the National Research Council, Ottawa, Canada (4). A 1.25-ml aliquot (∼250 μg) of this preparation was sent to Cocalico Biologicals, Inc. (Reamstown, Pa.) for production of rabbit antiserum. Briefly, for immunization, 100 μg of antigen emulsified in Freund's complete adjuvant was injected subcutaneously, and this was followed by three booster doses of 50 μg of antigen mixed with Freund's incomplete adjuvant given at 2-week intervals. The resulting antiserum was designated anti-cLst.
The second rabbit antiserum (designated HA7) was raised as described above against rLst prepared in the Rest laboratory, as described below.
Cloning of lst from N. meningitidis MC58⊄3.
The lst gene was amplified from a single colony of N. meningitidis MC58⊄3 by using the 5′ primer lst for (CGTAGGCATATGGGCTTGAAAAAGGCTTG) and the 3′ primer lst rev (TCTCGAGGTAATTTTTATCGTCAAATGTCAAA). The forward and reverse primers incorporated an NdeI site at the 5′ terminus and an XhoI site at the 3′ terminus of lst, respectively, as indicated by underlining. The resulting PCR product was gel purified, digested with NdeI and XhoI, and ligated into expression vector pTyB1 (NEB) that was digested with the same two enzymes. In the pTyB1expression vector Lst is fused at its C terminus to an affinity intein tag that is self-cleaved after binding to a chitin column in the presence of dithiothreitol (DTT), which allows Lst purification in the absence of a separate protease. The resulting clone (pSSLst) was electroporated into E. coli strain XL-1 Blue (Stratagene, La Jolla, Calif.) and selected on Luria broth (LB) plates containing 100 μg of ampicillin per ml and 10 μg of tetracycline per ml. Positive clones were verified by restriction analysis and sequencing (data not shown).
Expression, induction, and purification of rLst.
Purified pSSLst was electroporated into E. coli strain ER2566 (New England Biolab, Beverley, Mass.). Positive clones were identified by growth on LB containing 100 μg of ampicillin per ml and by restriction analysis of pSSLst. For expression of Lst, E. coli ER2566 harboring pSSLst was grown in 500 ml of LB containing 100 μg of ampicillin per ml at 37°C to an A600 of 0.5 to 0.6. Protein expression was induced by addition of 0.5 mM isopropyl-1-thio-β-d-galactopyranoside, and growth was continued for 5 h at the ambient temperature. Bacteria were harvested by centrifugation at 4,100 × g for 15 min. The supernatant was discarded, and the pellet was stored at −20°C.
The frozen pellet was thawed on ice and resuspended in 20 ml of suspension buffer (1.0 M NaCl, 0.02 M Tris-HCl [pH 8.0], 1.0 mM EDTA, 0.1% Triton X-100, protease inhibitor mixture [Sigma]). The suspension was sonicated on ice for 5 min in 30-s intervals and then centrifuged for 15 min at 23,000 × g at 4°C. The supernatant was collected and applied to a 5-ml chitin bead column that was preequilibrated with suspension buffer. After the sample passed through the column, the column was washed with 20 column volumes of the same buffer without protease inhibitors. The resin was then quickly washed with 3 column volumes of the same buffer containing 0.05 M DTT and protease inhibitors, and the effluent was discarded. The column was clamped at both ends, and on-column cleavage of the intein tag from the fusion protein was performed by incubating the column at 16°C for 18 h. Purified protein was eluted in 4 column volumes of the same buffer containing protease inhibitors and no DTT. The eluate (rLst) was concentrated by centrifugation with a Centricon 30 filtration apparatus (Bio-Rad), dialyzed for 3 h at 4°C against phosphate-buffered saline (PBS) containing 0.1% Triton X-100, divided into aliquots, and stored at −80°C.
Generation of HA7 antiserum.
Concentrated rLst was electrophoresed (4°C overnight at 30 V) on a nondenaturing preparative gel consisting of a 4% stacking gel in 1 M Tris-HCl (pH 6.8) and a 10% separating gel in 1 M Tris-HCl (pH 8.8) in a Tris-glycine (pH 8.3, 0.05 M) running buffer. A vertical strip was cut out from one side of the gel and quickly stained with Coomassie brilliant blue (CBB) to locate protein bands. Two horizontal strips, which aligned with the only two major proteins on the CBB-stained vertical strip, were excised from the gel, individually homogenized in PBS containing 0.1% Triton X-100, and tested for sialyltransferase activity as described above. The remainder of each strip was sent to Cocalico Biologicals, Inc., for production of rabbit antiserum as described above. The resulting antiserum was designated HA7.
Preparation of subcellular fractions.
Cellular fractions were prepared in the Judd laboratory by using a modification of the procedure of Osborn and Munson (21).
(i) Growth conditions.
Bacteria were grown for 16 h on clear typing medium (29). Cells were harvested and resuspended to an A600 of 1.0 in Dulbecco's PBS. Sixty-five microliters of the suspension was inoculated into 100 ml of GC broth plus supplement [200 ng of cocarboxylase per ml, 20.8 μg of Fe(NO3)3 · 9H2O per ml, 100 μg of l-glutamine per ml, 20 mM d-glucose] in a 250-ml sidearm flask and incubated with shaking at 37°C until the culture reached the mid-log growth phase. Then 0.5 ml of the mid-log-phase cells was inoculated into 250 ml of GC broth in a 1-liter sidearm flask and incubated with shaking until the culture again reached the mid-log growth phase. Bacteria were harvested by centrifugation at 12,000 × g for 10 min at room temperature.
(ii) Isolation of inner and outer membranes.
Pellets of whole cells, isolated as described above, were resuspended in 6.25 ml (per 250 ml of culture) of 200 mM Tris-HCl (pH 8.0) and diluted with an equal volume of ice-cold 1 M sucrose-200 mM Tris-HCl (pH 8.0). The following prechilled reagents were added sequentially with vortexing and mixing: 25 μl of 250 mM EDTA; 100 μl of N-acetylmuramidase (5 mg/ml in H2O distilled three times; mutanolysin; catalog no. M9901; Sigma); and 12.5 ml of 4°C distilled H2O added forcefully by pipetting. The preparation was gently shaken overnight at 4°C. Cells were then sonicated by using four 20-s bursts at 50% power with a Fisher 550 Sonic Dismembranator (Fisher Scientific). Unbroken cells and cell debris were removed by centrifugation at 12,000 × g for 10 min. The supernatant was then decanted into fresh centrifuge tubes and centrifuged at 12,000 × g for 30 min. The supernatant was then ultracentrifuged at 240,000 × g for 2 h at 4°C in a Ti80 rotor (Beckman). Pellets were resuspended in 1 ml of 18% (wt/wt) sucrose.
A step gradient was layered from bottom to top in 37-ml polypropylene tubes by adding the following sucrose solutions (all contained 1 mM EDTA and 200 μM DTT): 6 ml of 60% (wt/wt) sucrose overlaid with 5 ml each of 55, 50, 45, 35, 25, and 20% (wt/wt) sucrose. One milliliter of the membrane fraction was layered on top of the gradient and centrifuged in an SW28 rotor (Beckman) at 80,000 × g for 48 h at 4°C. Following centrifugation, 1-ml fractions were collected from the top of the gradient with a peristaltic pump. The fractions were analyzed by refractometry and by determining A280. Absorbing fractions in the density range from ∼1.15 to ∼1.26 g/ml were pooled, dialyzed, lyophilized, resuspended in 1 ml of 18% (wt/wt) sucrose, and layered on a second step gradient constructed by using 5.5 ml each of 60 and 55% sucrose and 5 ml each of 50, 45, 41, 37, and 33% sucrose. One-milliliter fractions were collected from the top of the gradient with a peristaltic pump.
To confirm separation and to identify inner and outer membrane fractions, all fractions were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and stained with CBB and silver. Only outer membrane fractions showed LOS staining. Western blots of separated fractions were probed with antibodies to known outer membrane markers (Por, Rmp, Lip, and MtrC). Only outer membrane fractions reacted with these antibodies. Representative fractions from inner and outer membrane peaks were tested for inner membrane markers, including several dehydrogenases (18), heme-containing proteins, and cytochrome oxidase activity (28). Only inner membrane fractions showed dehydrogenase and cytochrome oxidase activities and possessed heme-containing proteins. These observations have been reported by Judd and colleagues (8).
Fractions from the centers of the inner and outer membrane peaks were either tested for Lst activity or solubilized (1:1, vol/vol) in 2× SDS-PAGE solubilizing solution and used in SDS-PAGE and Western blot studies.
(iii) Isolation of periplasm, cytoplasm, and culture supernatant.
Periplasmic contents were isolated as described by Judd and Porcella (7) from cells grown as described above. Cells were harvested at an A600 of 0.68. Chloroform incubation was performed at 4°C to preserve Lst enzymatic activity. The periplasmic isolate was either tested for Lst activity or solubilized (1:1, vol/vol) in 2× SDS-PAGE solubilizing solution and used in SDS-PAGE and Western blot studies. Cytoplasmic contents were isolated from cells grown on plates and harvested at an A600 of 0.8 in PBS as described above. The cellular pellets were each resuspended in 100 μl of Dulbecco's PBS and subjected to four 20-s bursts at 50% power by using the microtip of a Fisher 550 Sonic Dismembranator. Cellular debris was removed by centrifugation at 12,000 × g for 10 min. The supernatant was aspirated and ultracentrifuged at 130,000 × g for 1 h. The clarified supernatant was collected and either tested for sialyltransferase activity or solubilized (1:1, vol/vol) in 2× SDS-PAGE solubilizing solution and used in SDS-PAGE and Western blot studies.
Culture supernatants were isolated from cells grown as described above. Whole cells and cellular debris were removed by centrifugation at 12,000 × g for 10 min at 4°C. The supernatant was aspirated and subjected to ultracentrifugation at 225,000 × g for 4 h at 4°C. The clarified supernatant was either tested for Lst activity or solubilized (1:1, vol/vol) in 2× SDS-PAGE solubilizing solution and used in SDS-PAGE and Western blot studies.
Measurement of sialyltransferase activity.
Sialyltransferase activity was determined by measuring the incorporation of radiolabeled N-acetylneuraminic acid (NANA) into purified gonococcal LOS, essentially as described by Mandrell et al. (13). Typically, 50-μl reaction mixtures contained 0.1 M sodium phosphate buffer (pH 6.8), 0.1% Triton X-100, 0.8% bovine serum albumin, 0.85 nmol of cold CMP-NANA, 0.094 nmol (0.03 μCi) of [14C]CMP-NANA (Amersham, Piscataway, N.J.), 3.8 nmol of LOS (purified from N. gonorrhoeae strain F62), and various amounts of Lst. The reaction mixtures were incubated at 37°C for 15 min, and the reactions were stopped by addition of 0.5 ml of 5% phosphotungstic acid-15% trichloroacetic acid. Precipitates were washed, solubilized, and counted. Subcellular fractions made in the Judd laboratory were blinded and sent to the Rest laboratory for measurement of sialyltransferase activity.
Inhibition of sialyltransferase activity by anti-rLst antibody HA7.
Aliquots of rLst were incubated in 20-μl mixtures with different dilutions of HA7 antibody for 15 min at room temperature. Ten-microliter aliquots of each mixture were removed, and their Lst activities were measured. As a positive control, rLst was incubated for the same period of time in antibody dilution buffer (PBS containing 1% bovine serum albumin) in the absence of HA7. Lst was also tested for enzymatic activity after incubation with preimmune serum, as indicated below.
Immunoprecipitation of Lst.
A Seize X protein A immunoprecipitation kit from Pierce (Rockford, Ill.) was used as recommended in the manufacturer's protocol. A 1:1,000 dilution of preimmune serum and anti-rLst antiserum HA7 were used in these assays. Twenty-five-microliter aliquots of the collected fractions were analyzed by Western blotting by using HA7 (1:25,000 dilution) as the probe, and 10-μl aliquots were tested for sialyltransferase activity as described above.
SDS-PAGE and Western blotting.
For analyzing subcellular fractions, SDS-PAGE and Western blotting were preformed as detailed by Judd (6). The anti-Lst antisera, anti-cLst, and HA7 were each used at a 1:10,000 dilution.
Whole-cell binding assays.
To assess the exposure of Lst on the bacterial surface, N. meningitidis MC58⊄3 and N. gonorrhoeae strains F62 and ST01 were grown in GC broth to an A550 of 1.0, as described above. Aliquots were collected in microcentrifuge tubes, pelleted, and resuspended in 1 ml of PBSG (Dulbecco’s PBS plus 0.1% [wt/vol] gelatin) containing 0.001% Ca2+, Mg2+, and various dilutions of preadsorbed rabbit anti-Lst antiserum HA7. Samples were mixed end over end for 30 min at room temperature and centrifuged at 10,000 × g for 1 min. The pellets were washed twice with 1 ml of PBS containing 0.05% Tween 20, and the final pellets were suspended in 1 ml of PBS containing 0.05% Tween 20 with a 1:5,000 dilution of protein A-conjugated alkaline phosphatase (Calbiochem). Samples were mixed end over end for 1 h at room temperature and centrifuged. The pellets were washed twice as described above, suspended in 210 μl of a solution containing 1 mg of p-nitrophenyl phosphate (Sigma) per ml in 10% sodium carbonate-1 mM MgCl2, and left at room temperature until color developed. The reactions were terminated with 90 μl of 10 N NaOH. Samples were centrifuged as described above, and the optical densities at 405 nm of the supernatants were measured.
RESULTS
Production and characterization of anti-cLst antiserum.
For detection of Lst in subcellular fractions, we immunized rabbits with c-Myc-tagged Lst originating from N. meningitidis MC58⊄3 as described in Materials and Methods. The resulting antiserum, anti-cLst, was used to probe whole-cell blots of N. gonorrhoeae F62 and its isogenic lst mutant, ST01. At a 1:10,000 dilution the antiserum detected a 43-kDa band in whole-cell extracts of F62 but not in ST01 (Fig. 1A, right panel, lanes F62 WC and ST01 WC). This indicated that the anti-meningococcal Lst antiserum detected gonococcal Lst, which was not unexpected considering that the Lst molecules of N. gonorrhoeae and N. meningitidis strains share ∼95% identity. Proteins with molecular masses of ∼90 and ∼70 kDa were also detected with this antiserum in whole-cell blots of N. gonorrhoeae F62 and ST01. In addition, the anti-cLst bound a protein with a molecular mass of ∼100 kDa in whole-cell blots of N. meningitidis MC58⊄3 (Fig. 1B, right panel, lane WC). Reactivity with these proteins probably represented cross-reactivity of the antiserum and indicated the presence of contaminating proteins that eluted with the recombinant cLst during purification. However, it was clear from the results that the antiserum could differentially bind a 43-kDa protein in strains positive for Lst, which would allow its use in detection of Lst in subcellular fractions.
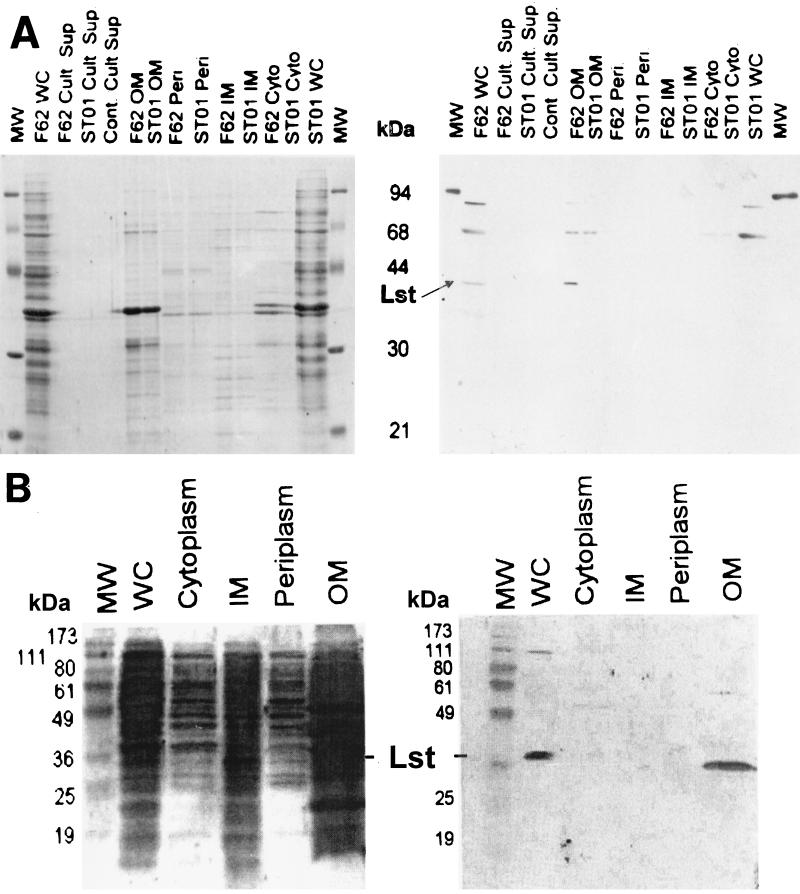
FIG. 1.
SDS-PAGE and Western blot analyses of subcellular fractions of N. gonorrhoeae strains F62 and ST01 and N. meningitidis strain MC58⊄3. (A, left panel) Whole-cell lysates (WC), culture supernatants (Cult Sup), isolated outer membranes (OM), isolated periplasm (Peri), isolated inner membranes (IM), and isolated cytoplasm (Cyto) from N. gonorrhoeae strains F62 and ST01 were separated in an SDS-12.5% PAGE gel and stained with CBB. Lanes Cont Cult Sup contained sterile growth medium. Lanes MW contained molecular mass markers. (A, right panel) Western blot of the SDS-PAGE gel shown in the left panel probed with anti-cLst antiserum, as described in Materials and Methods. The position of the 43-kDa Lst is indicated by an arrow. (B) Whole-cell (WC), cytoplasmic, inner membrane (IM), periplasmic, and outer membrane (OM) fractions of N. meningitidis MC58⊄3 were separated in an SDS-12% PAGE gel and transferred to a polyvinylidene difluoride membrane. (Right panel) Polyvinylidene difluoride membrane probed with anti-cLst, preadsorbed with a lysate of E. coli DH5α. (Left panel) Blot reblocked with 0.02% Tween and stained with India ink. Lane MW contained prestained markers. The position of Lst at ∼43 kDa is indicated.
Immunodetection of Lst in subcellular fractions of N. gonorrhoeae strains F62 and ST01 and N. meningitidis strain MC58⊄3.
Subcellular fractions of N. gonorrhoeae F62 and ST01 and N. meningitidis MC58⊄3 were prepared as described in Materials and Methods. Approximately 100 to 200 μg of each fraction was analyzed by SDS-PAGE and subjected to Western blot analysis by using a 1:10,000 dilution of anti-cLst antiserum as the probe (Fig. 1). A 43-kDa protein was detected in the outer membrane fraction but not in the inner membrane, periplasm, cytoplasm, or culture supernatant fraction of N. gonorrhoeae F62. As expected, the antiserum did not react with a 43-kDa protein in any of the cellular fractions of the lst mutant ST01.
Since N. meningitidis serogroups B and C can utilize endogenous (i.e., internal) sources of CMP-NANA to sialylate their LOS, we were curious to know whether the subcellular distribution of Lst in N. meningitidis MC58⊄3, an acapsulate mutant serogroup B strain, was the same as that observed in N. gonorrhoeae. As shown in Fig. 1B, Lst was detected only in whole-cell preparations (lane WC) and in outer membrane preparations (lane OM) of N. meningitidis, while other subcellular fractions were devoid of an ∼43-kDa reactive protein. These results showed that Lst localizes to the outer membrane in both N. gonorrhoeae and N. meningitidis, in contrast to the hypothesized inner membrane distribution of LPS glycosyltransferases (20).
Measurement of Lst enzymatic activity in cellular fractions of N. gonorrhoeae strains F62 and ST01.
To confirm the results of the Western blot analyses, we tested the subcellular fractions of F62 and ST01 for sialyltransferase enzymatic activity. Prior to testing, each fraction was blind labeled in order to obtain an unbiased evaluation of the results. Consistent with our immunodetection studies, sialyltransferase activity was detected almost exclusively in the outer membrane fraction of F62 (Table 1). A trace of activity was also detected in the inner membrane fraction, indicating the higher sensitivity of the enzyme assay than of the Western blot analysis. This activity was about 2.4% of that found in the outer membrane and could have been due to fragments of membrane that did not separate during isolation. It is also possible that a small amount of Lst is present in the inner membrane before transport to the outer membrane. These results support our conclusion that the 43-kDa protein detected in the outer membrane fractions of N. gonorrhoeae F62 and N. meningitidis MC58⊄3 was indeed Lst.
TABLE 1.
Sialyltransferase activity in subcellular fractions of N. gonorrhoeae strains F62 and ST01
| Fraction | Sialyltransferase activity (cpm/μg of protein)a
|
|
|---|---|---|
| Strain F62 | Strain ST01 | |
| Culture supernatant | 0 | 0 |
| Control culture supernatant | 0 | 0 |
| Outer membrane | 4,660 ± 168 | 0 |
| Periplasm | 0 | 0 |
| Inner membrane | 127 ± 2.8 | 0 |
| Cytoplasm | 0 | 0 |
Fraction samples were assayed as described in Materials and Methods. The sialyltransferase activity values were subtracted from background values and are representative of the results of at least three independent experiments conducted in duplicate by using one set of fractions. All fractions were measured at a protein concentration of 0.6 to 0.66 μg/μl.
Generation and characterization of HA7 anti-Lst antiserum.
We next investigated the possible surface exposure of Lst on N. meningitidis and N. gonorrhoeae. To facilitate this study, we generated antiserum that bound native Lst. The c-Myc-tagged rLst used to raise antiserum to cLst exhibited minimal sialyltransferase activity (data not shown), indicating that it contained little native Lst. In addition, preliminary studies indicated that the anti-cLst antiserum did not detect Lst on whole bacteria, nor did it immunoprecipitate enzymatically active Lst (data not shown). Therefore, we attempted to raise new antiserum that reacted with native Lst. For this endeavor, the lst gene of N. meningitidis MC58⊄3 was cloned and expressed in E. coli by using the construct pSSLst, which included the open reading frame of lst and a 3′ sequence encoding a self-cleaving intein tag to facilitate purification, as described in Materials and Methods. This rLst preparation was analyzed by SDS-PAGE and Western blotting by using anti-cLst as the probe and was also tested for sialyltransferase activity. The rLst was identified as a 43-kDa protein by Western blot analysis (it was accompanied by 70- and 100-kDa proteins) and possessed significant sialyltransferase activity (data not shown). To further purify rLst for immunization, it was subjected to native PAGE as described in Materials and Methods and used to raise rabbit antiserum. In a Western blot analysis, the resulting antiserum, designated HA7, recognized Lst and, to a lesser degree, proteins with molecular masses of ∼70 and ∼100 kDa in whole-cell extracts of N. gonorrhoeae (data not shown).
Inhibition of Lst activity and immunoprecipitation of native Lst by HA7 antiserum.
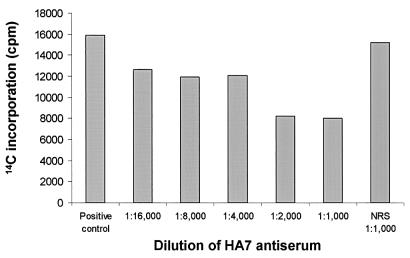
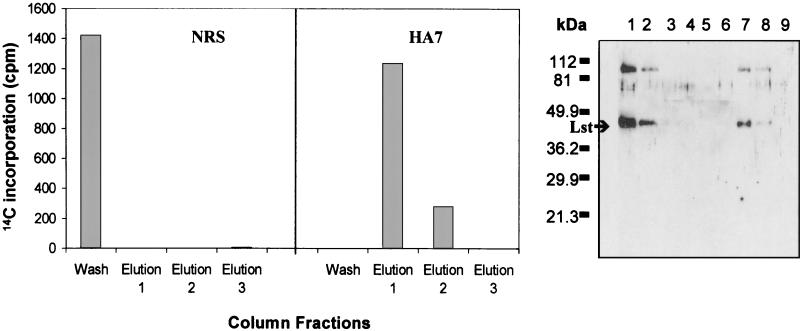
As a measure of its ability to bind active rLst, we used HA7 to inhibit Lst sialyltransferase activity and to immunoprecipitate rLst. rLst was incubated with various dilutions of HA7 serum and subsequently tested for enzymatic activity. Enzymatic activity decreased by approximately 50% after rLst was incubated with a 1:2,000 or 1:1,000 dilution of HA7 (Fig. 2), and Lst protein and enzymatic activity were immunoprecipitated with HA7 immunoglobulin G (IgG) immobilized on a protein A column. A 1:1,000 dilution of preimmune serum had no significant effect on Lst activity (Fig. 2). Moderate inhibition of Lst activity by the antiserum suggested that native epitopes were being bound but not in a region(s) important for catalysis. We next immunoprecipitated Lst protein with HA7 IgG immobilized on a protein A column. As a control, a second protein A column was prepared by using IgG from rabbit preimmune serum. Whereas Lst applied to the protein A column prepared with preimmune serum was found predominately in the column flowthrough, Lst protein and enzymatic activity were bound tightly to the column prepared with HA7 (Fig. 3). The results indicated that HA7 antiserum contained antibodies against native Lst epitopes and might be helpful in demonstrating the surface exposure of Lst in N. gonorrhoeae or N. meningitidis.
FIG. 2.
Inhibition of rLst activity by HA7 anti-rLst antiserum. rLst was incubated with different dilutions of HA7 antiserum and tested for sialyltransferase activity as described in Materials and Methods. The positive control was Lst mixed with assay buffer. Sialyltransferase activity was also measured after incubation with normal rabbit antiserum (NRS) isolated from rabbits prior to immunization. The data are representative of the results of two experiments.
FIG. 3.
Affinity purification of native rLst by HA7 anti-rLst antiserum. rLst was applied to a protein A column containing bound antibodies from HA7 antiserum or normal rabbit serum (NRS). The column was washed, and rLst was dissociated from the column with a three-step elution procedure as described in Materials and Methods. The wash and three elution fractions were tested for Lst sialyltransferase activity (left panel) and by Western analysis for rLst protein (right panel) as described in Materials and Methods. (Right panel) Lane 1, rLst; lane 2, NRS column, wash; lane 3, NRS column, elution 1; lane 4, NRS column, elution 2; lane 5, NRS column, elution 3; lane 6, HA7 column, wash; lane 7, HA7 column, elution 1; lane 8, HA7 column, elution 2; lane 9, HA7 column, elution 3. The positions of Lst and molecular mass markers are indicated on the left of the Western blot.
HA7 binding to whole cells.
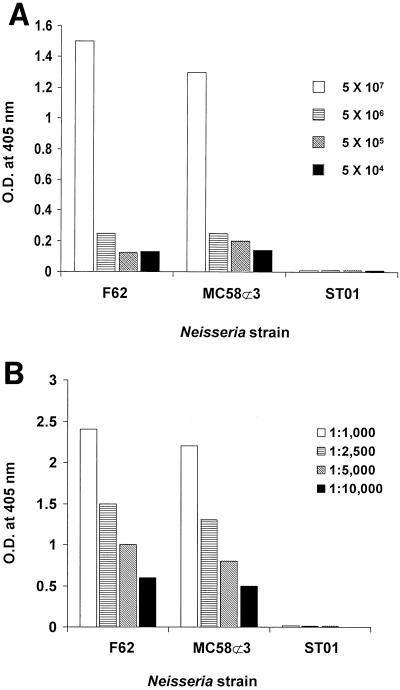
To determine if Lst is exposed on the surface of whole cells, N. gonorrhoeae F62 or N. meningitidis MC58⊄3 was incubated with HA7 antiserum previously adsorbed with our lst mutant, ST01. Antibody binding was detected by using protein A conjugated to alkaline phosphatase. At a 1:2,500 dilution, HA7 antibody bound to various numbers of bacteria in a dose-dependent manner (Fig. 4A). Similarly, various concentrations of HA7 antibody bound to a constant number of Neisseria cells in a dose-dependent manner (Fig. 4B). Very similar results were observed for N. meningitidis and N. gonorrhoeae. As expected, HA7 did not bind to lst mutant ST01 at the lowest dilution of antibody or the highest number of bacteria used. Thus, at least some portion of Lst is exposed on the surface of N. gonorrhoeae and N. meningitidis.
FIG. 4.
HA7 anti-Lst antiserum binding to whole cells. (A) Different concentrations of N. gonorrhoeae F62 or ST01 (lst−) and N. meningitidis MC58⊄3 were mixed with HA7 antiserum at a final dilution of 1:2,500 and washed, and the amount of antibody bound was detected by incubation with protein A-alkaline phosphatase as described in Materials and Methods. (B) Bacteria (5 × 107 cells) were mixed with different dilutions of HA7 antiserum and examined for bound antibodies as described above. The data are representative of the results of two experiments.
DISCUSSION
The pathogenic neisseriae sialylate their surfaces with an LOS-specific α-2,3-sialyltransferase (Lst) (24, 26). Characterization of Lst has revealed that it is a monomeric, 43-kDa protein that is antigenically stable and constitutively expressed (11). Several properties of Lst, including its association with membrane fragments of whole cells of N. meningitidis and recombinant E. coli, have suggested a membrane location for this enzyme (2-4). In the present study we expanded the characterization of Lst by demonstrating its outer membrane location and surface exposure in whole cells of N. gonorrhoeae F62 and N. meningitidis MC58⊄3.
Cloning of lst and isolation of its gene product have facilitated construction of a defined lst insertion mutant and generation of Lst-specific antisera (4; Rest et al., unpublished data). To localize Lst in subcellular fractions of N. gonorrhoeae F62 and N. meningitidis MC58⊄3, partially purified c-Myc-tagged rLst was used to generate rabbit anti-cLst antiserum. We also used the cloned, insertionally inactivated lst gene of N. meningitidis MC58⊄3 to produce N. gonorrhoeae ST01, an isogenic lst mutant of N. gonorrhoeae F62, as a control. When used to probe Western blots of whole cells, anti-cLst antiserum reacted with a 43-kDa protein in whole-cell and outer membrane preparations of N. gonorrhoeae F62 and N. meningitidis MC58⊄3 and not in cellular preparations of the Lst mutant, ST01. To confirm these results, we tested the subcellular preparations of N. gonorrhoeae F62 and ST01 for the ability to sialylate purified LOS in vitro by using radiolabeled NANA. In agreement with our Western blot studies, Lst activity was detected mainly in the outer membrane fraction of F62. The inner membrane fraction also contained a bit of Lst activity that was barely greater than the background level. We attribute this activity either to unseparated membrane fragments (20) or to Lst that is en route to the outer membrane. The results of this investigation demonstrated an outer membrane location for Lst in N. gonorrhoeae and N. meningitidis. The dependence on an external source of CMP-NANA has long suggested this distribution for Lst in N. gonorrhoeae and further substantiates the observation that CMP-NANA is absorbed by intact N. gonorrhoeae (2). Endogenous sialylation of N. meningitidis LOS by some serogroups, due to internal synthesis of CMP-NANA, suggested that Lst in these serogroups might face the inside of the cell, perhaps on the inside of the outer membrane. Thus, Lst would still have access to intracellular CMP-NANA depending on its orientation. Studies of the physiology of capsule formation by the inner membrane α-2,8-polysialyltransferase suggested that activated NANA could be available near the cytoplasmic or periplasmic face of the inner membrane (14, 15).
The outer membrane distribution of Lst prompted us to investigate if it was surface exposed. In preliminary studies we attempted to use anti-cLst antiserum to show binding to whole cells, but we were unsuccessful because the antiserum did not recognize native Lst. Thus, we generated antiserum HA7, which recognized native Lst. Binding to native Lst was assessed by specific enzyme inhibition and immunoprecipitation. Using protein A beads conjugated to alkaline phosphatase as a method of detection and adsorbed antiserum, we were able to show that HA7 binds to whole cells of N. gonorrhoeae F62 and of N. meningitidis MC58⊄3, indicating that some part of Lst is exposed on the surface of Neisseria cells.
Our data show that Lst is an outer membrane, surfaced-exposed enzyme in pathogenic neisseriae. Future experiments will concentrate on investigating (i) the serum and mucosal humoral responses to Lst and (ii) the ability of Lst antibodies to facilitate or enhance opsonophagocytosis or complement-mediated killing of neisseriae.
Acknowledgments
We thank Deborah Nycz and Jenne Quick for technical support in Montana.
R.C.J. was funded by National Institutes of Health grants R01 AI21236 and R01 AI47254 and by University of Montana grant UMG1646. R.F.R. was funded by National Institutes of Health grant R01 AI33505.
Editor: D. L. Burns
REFERENCES
- 1.Frangipane, J. V., and R. F. Rest. 1993. Anaerobic growth and cytidine 5′-monophospho-N-acetylneuraminic acid act synergistically to induce high-level serum resistance in Neisseria gonorrhoeae. Infect. Immun. 61:1657-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao, L., L. Linden, N. J. Parsons, J. A. Cole, and H. Smith. 2000. Uptake of metabolites by gonococci grown with lactate in a medium containing glucose: evidence for a surface location of the sialyltransferase. Microb. Pathog. 28:257-266. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert, M., A. M. Cunningham, D. C. Watson, A. Martin, J. C. Richards, and W. W. Wakarchuk. 1997. Characterization of a recombinant Neisseria meningitidis alpha-2,3-sialyltransferase and its acceptor specificity. Eur. J. Biochem. 249:187-194. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert, M., D. C. Watson, A. M. Cunningham, M. P. Jennings, N. M. Young, and W. W. Wakarchuk. 1996. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J. Biol. Chem. 271:28271-28276. [DOI] [PubMed] [Google Scholar]
- 5.Jennings, M. P., D. W. Hood, I. R. Peak, M. Virji, and E. R. Moxon. 1995. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol. Microbiol. 18:729-740. [DOI] [PubMed] [Google Scholar]
- 6.Judd, R. C. 1986. Evidence for N-terminal exposure of the PI class of protein I of Neisseria gonorrhoeae. Infect. Immun. 54:408-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judd, R. C., and S. F. Porcella. 1993. Isolation of the periplasm of Neisseria gonorrhoeae. Mol. Microbiol. 10:567-574. [DOI] [PubMed] [Google Scholar]
- 8.Judd, R. D., C. E. Tilly, J. Smith, and D. S. Manning. 1996. Isolation of the outer membrane of Neisseria gonorrhoeae, p. 39-40. In C. D. Deal (ed.), The pathogenic Neisseria. National Institutes of Health, Bethesda, Md.
- 9.Kahler, C. M., L. E. Martin, G. C. Shih, M. M. Rahman, R. W. Carlson, and D. S. Stephens. 1998. The (α2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect. Immun. 66:5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotwel, G. 1997. Microorganisms and their interaction with the immune system. J. Leukoc. Biol. 62:415-429. [DOI] [PubMed] [Google Scholar]
- 11.Mandrell, R. E., J. M. Griffiss, H. Smith, and J. A. Cole. 1993. Distribution of a lipooligosaccharide-specific sialyltransferase in pathogenic and non-pathogenic Neisseria. Microb. Pathog. 14:315-327. [DOI] [PubMed] [Google Scholar]
- 12.Mandrell, R. E., A. J. Lesse, J. V. Sugai, M. Shero, J. M. Griffiss, J. A. Cole, N. J. Parsons, H. Smith, S. A. Morse, and M. A. Apicella. 1990. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J. Exp. Med. 171:1649-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandrell, R. E., H. Smith, G. A. Jarvis, J. M. Griffiss, and J. A. Cole. 1993. Detection and some properties of the sialyltransferase implicated in the sialylation of lipopolysaccharide of Neisseria gonorrhoeae. Microb. Pathog. 14:307-313. [DOI] [PubMed] [Google Scholar]
- 14.Masson, L., and B. E. Holbein. 1985. Influence of nutrient limitation and low pH on serogroup B Neisseria meningitidis capsular polysaccharide levels: correlation with virulence for mice. Infect. Immun. 47:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masson, L., and B. E. Holbein. 1985. Role of lipid intermediate(s) in the synthesis of serogroup B Neisseria meningitidis capsular polysaccharide. J. Bacteriol. 161:861-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGee, D. J., and R. F. Rest. 1996. Regulation of gonococcal sialyltransferase, lipooligosaccharide, and serum resistance by glucose, pyruvate, and lactate. Infect. Immun. 64:4630-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuinness, B. T., I. N. Clarke, P. R. Lambden, A. K. Barlow, J. T. Poolman, D. M. Jones, and J. E. Heckels. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514-517. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno, T., and M. Kageyama. 1978. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J. Biochem. (Tokyo) 84:179-191. [DOI] [PubMed] [Google Scholar]
- 19.Osborn, M. J. 1982-1983. Biosynthesis of the outer membrane of Salmonella. Harvey Lect. 78:87-103. [PubMed] [Google Scholar]
- 20.Osborn, M. J., J. E. Gardner, and E. Paris. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. J. Biol. Chem. 217:3972-3986. [PubMed] [Google Scholar]
- 21.Osborn, M. J., and R. Munson. 1974. Separation of the inner (cytoplasmic) and outer membrane of gram-negative bacteria. Methods Enzymol. 31:642-653. [DOI] [PubMed] [Google Scholar]
- 22.Parsons, N. J., G. J. Boons, P. R. Ashton, P. D. Redfern, P. Quirk, Y. Gao, C. Constantinidou, J. Patel, J. Bramley, J. A. Cole, and H. Smith. 1996. Lactic acid is the factor in blood cell extracts which enhances the ability of CMP-NANA to sialylate gonococcal lipopolysaccharide and induce serum resistance. Microb. Pathog. 20:87-100. [DOI] [PubMed] [Google Scholar]
- 23.Ram, S., F. G. Mackinnon, S. Gulati, D. P. McQuillen, P. Vogel, M. Frosch, C. Elkins, H.-K. Guttormsen, L. M. Wetzler, M. Oppermann, M. K. Pangburn, and P. A. Rice. 1999. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 36:915-928. [DOI] [PubMed] [Google Scholar]
- 24.Rest, R. F., and R. E. Mandrell. 1995. Neisseria sialyltransferases and their role in pathogenesis. Microb. Pathog. 19:379-390. [DOI] [PubMed] [Google Scholar]
- 25.Robertson, B. D., and T. F. Meyer. 1992. Genetic variation in pathogenic bacteria. Trends Genet. 8:422-426. [DOI] [PubMed] [Google Scholar]
- 26.Smith, H., J. A. Cole, and N. J. Parsons. 1992. The sialylation of gonococcal lipopolysaccharide by host factors: a major impact on pathogenicity. FEMS Microbiol. Lett. 79:287-292. [DOI] [PubMed] [Google Scholar]
- 27.Smith, H., N. J. Parsons, and J. A. Cole. 1995. Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb. Pathog. 19:365-377. [DOI] [PubMed] [Google Scholar]
- 28.Strange, J. C., and R. C. Judd. 1993. Characterization of heme-containing proteins of Neisseria gonorrhoeae, p. 417-423. In C. Conde-Glez (ed.), Eighth International Conference on Pathogenic Neisseria. Instituto National de Salud Publica, Cuernavaca, Mexico.
- 29.Swanson, J. 1978. Studies on gonococcus infection. XII. Colony color and opacity variants of gonococci. Infect. Immun. 19:320-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel, U., H. Claus, G. Heinze, and M. Frosch. 1999. Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect. Immun. 67:954-957. [DOI] [PMC free article] [PubMed] [Google Scholar]