Abstract
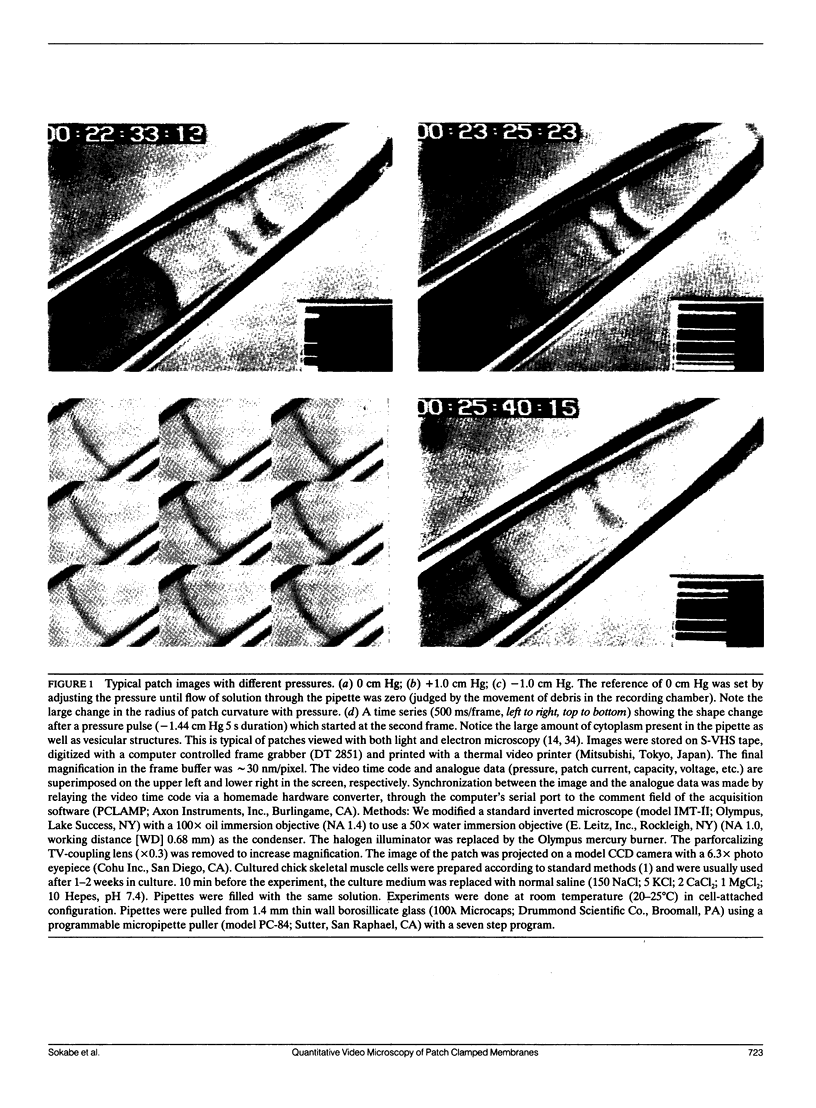
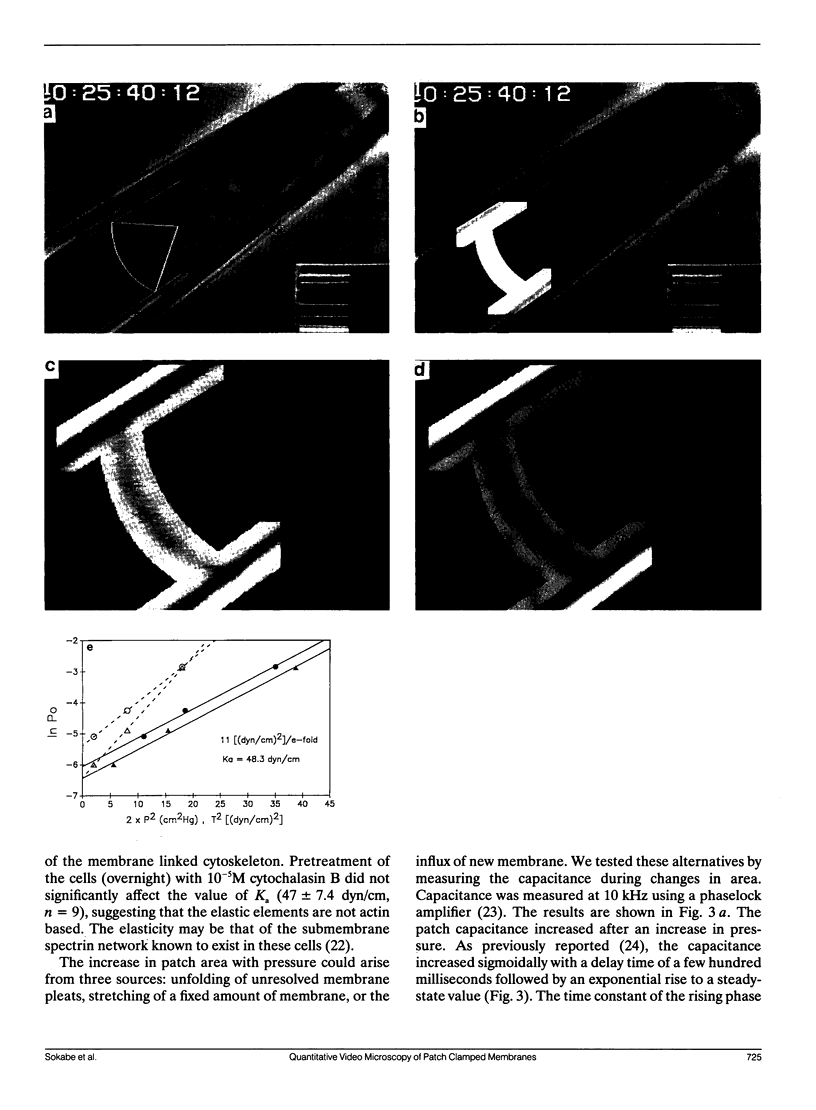
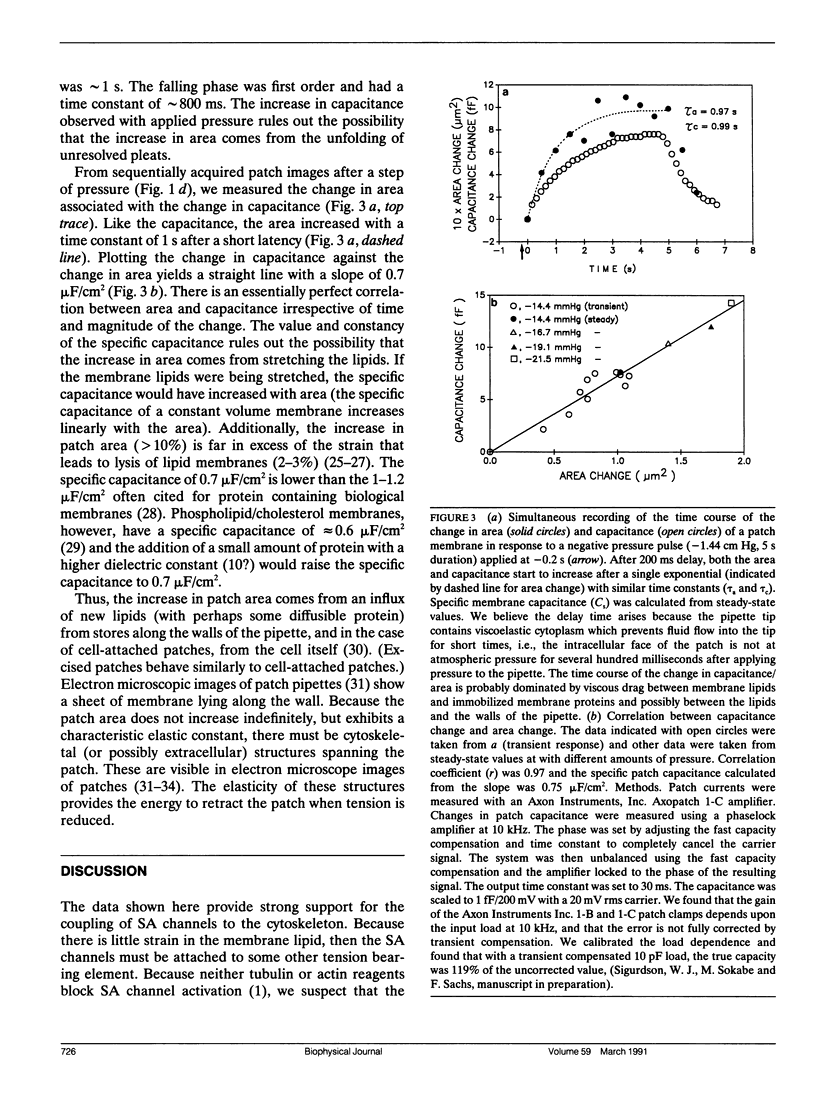
Membrane patches from chick skeletal muscle were stretched by applying controlled suction or pressure to the pipette. From images of the patch, the patch dimensions (area and radius of curvature) were computed by nonlinear regression of the images to a geometric model. With no applied pressure, patch membranes are nearly planar and normal to the wall of the pipette. With increasing pressure gradients, the patch bulges, the radius of curvature decreases, and the area increases. The patch capacitance changes in exact proportion to the change in area at a rate of 0.7 microF/cm2. The increase in area is due to a flow of lipid (with perhaps small amounts of diffusible protein) along the walls of the pipette into the patch. The flow is reversible with a relaxation of the pressure gradient. The area elastic constant of the membrane is approximately 50 dyn/cm, insensitive to cytochalasin B and probably represents the elasticity of the underlying spectrin/dystrophin network. Simultaneous measurements of stretch activated (SA) ion channel activity in the patch showed that the sensitivity of channels from different patches, although different when calculated as a function of applied pressure, was the same when calculated as a function of tension. Because patch lipid is free to flow, and hence stress-free in the steady state, SA channels must be activated by tension in the cytoskeleton.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brehm P., Kullberg R., Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol. 1984 May;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. E., Tang J. M., Rae J. L., Eisenberg R. S. A cation channel in frog lens epithelia responsive to pressure and calcium. J Membr Biol. 1986;93(3):259–269. doi: 10.1007/BF01871180. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Waugh R., Melnik L. Elastic area compressibility modulus of red cell membrane. Biophys J. 1976 Jun;16(6):585–595. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Needham D. Giant vesicle bilayers composed of mixtures of lipids, cholesterol and polypeptides. Thermomechanical and (mutual) adherence properties. Faraday Discuss Chem Soc. 1986;(81):267–280. doi: 10.1039/dc9868100267. [DOI] [PubMed] [Google Scholar]
- Gao X. Q., Sachs F. Improving performance of motorized slides for micromanipulation. J Neurosci Methods. 1989 Jun;28(3):225–227. doi: 10.1016/0165-0270(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M. C., Martinac B., Saimi Y., Culbertson M. R., Kung C. Ion channels in yeast. Science. 1986 Sep 12;233(4769):1195–1197. doi: 10.1126/science.2426783. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Kirber M. T., Walsh J. V., Jr, Singer J. J. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflugers Arch. 1988 Sep;412(4):339–345. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Milton R. L., Caldwell J. H. Na current in membrane blebs: implications for channel mobility and patch clamp recording. J Neurosci. 1990 Mar;10(3):885–893. doi: 10.1523/JNEUROSCI.10-03-00885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E., Sigurdson W. J. Stretch-inactivated ion channels coexist with stretch-activated ion channels. Science. 1989 Feb 10;243(4892):807–809. doi: 10.1126/science.2536958. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repasky E. A., Pollina C. M., Menold M. M., Hudecki M. S. Increased concentration of spectrin is observed in avian dystrophic muscle. Proc Natl Acad Sci U S A. 1986 Feb;83(3):802–806. doi: 10.1073/pnas.83.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Baroreceptor mechanisms at the cellular level. Fed Proc. 1987 Jan;46(1):12–16. [PubMed] [Google Scholar]
- Sachs F. Mechanical transduction in biological systems. Crit Rev Biomed Eng. 1988;16(2):141–169. [PubMed] [Google Scholar]
- Sivasubramanian N., Nayak D. P. Mutational analysis of the signal-anchor domain of influenza virus neuraminidase. Proc Natl Acad Sci U S A. 1987 Jan;84(1):1–5. doi: 10.1073/pnas.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M., Sachs F. The structure and dynamics of patch-clamped membranes: a study using differential interference contrast light microscopy. J Cell Biol. 1990 Aug;111(2):599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockbridge L. L., French A. S. Stretch-activated cation channels in human fibroblasts. Biophys J. 1988 Jul;54(1):187–190. doi: 10.1016/S0006-3495(88)82944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J., Steponkus P. L. The stress-strain relation of the plasma membrane of isolated plant protoplasts. Biochim Biophys Acta. 1981 May 20;643(3):663–668. doi: 10.1016/0005-2736(81)90363-1. [DOI] [PubMed] [Google Scholar]