Abstract
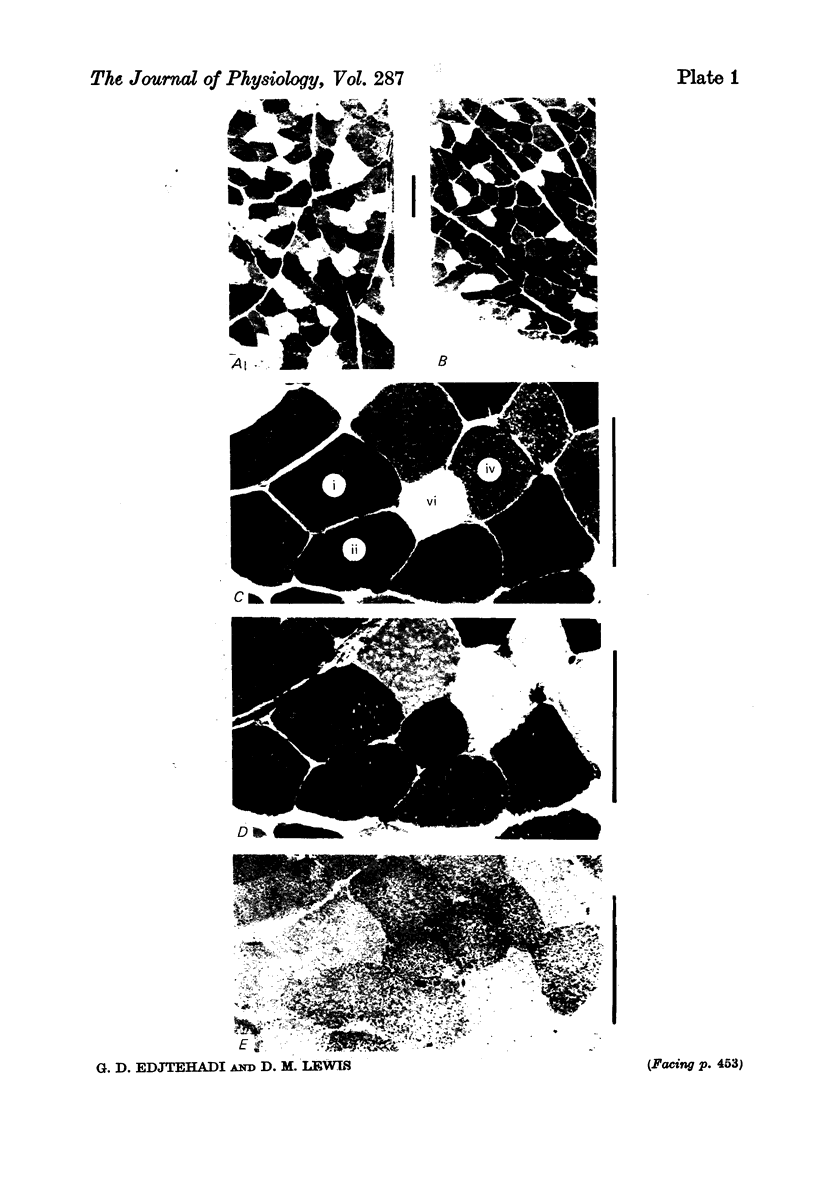
1. Serial sections of flexor digitorum longus muscle (f.d.l.) of the cat were examined histochemically for four enzyme systems: adenosine triphosphatase (ATPase) with alkaline and acid pre-incubation, phosphorylase and succinic dehydrogenase (SDHase).
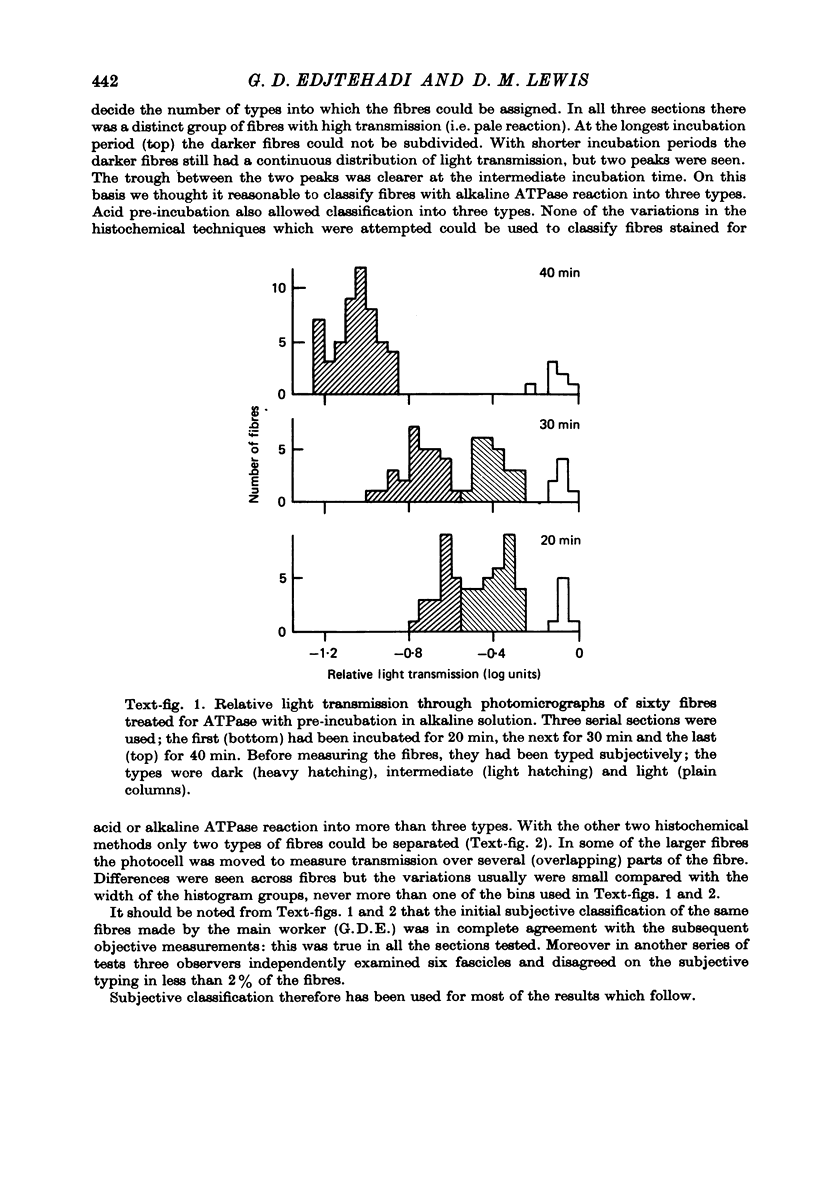
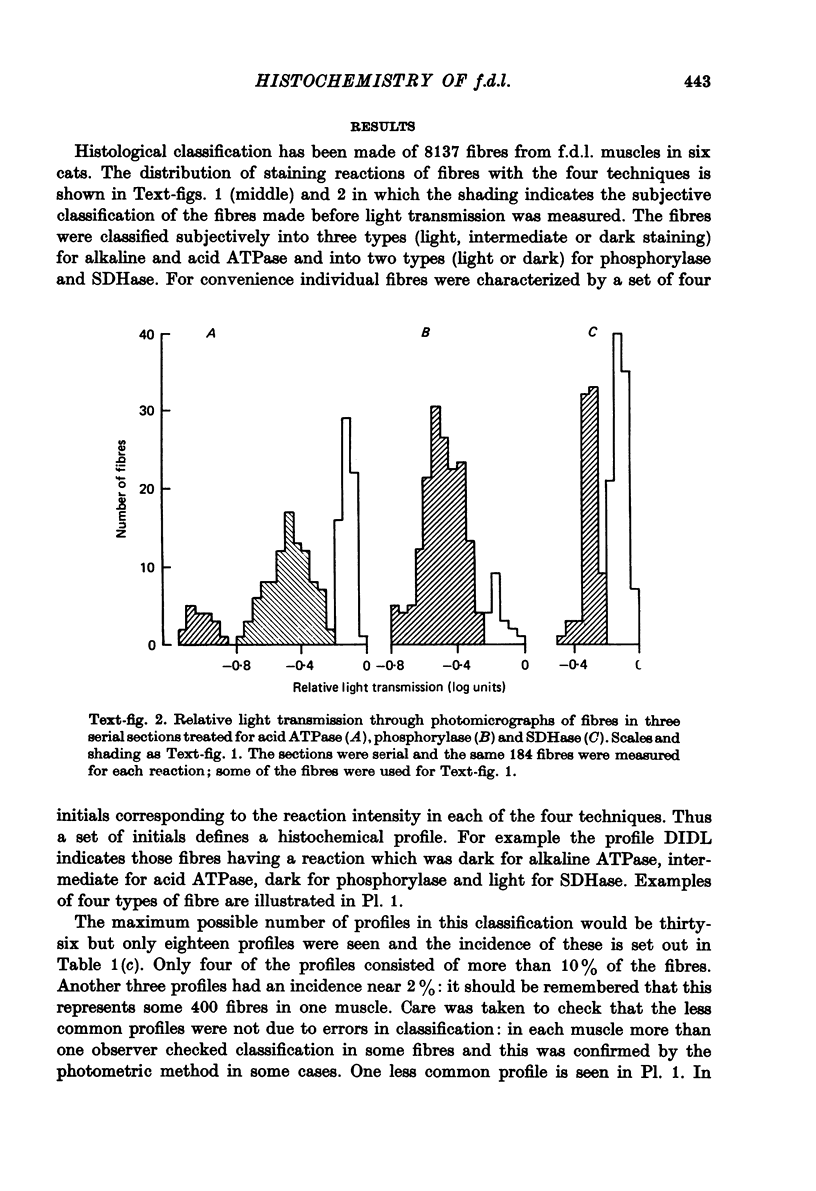
2. The number of types into which fibres should be divided was assessed by estimating enzyme reaction intensity from measurements of light transmission through photomicrographs. It was concluded that in general the enzyme reaction intensities of fibres were distributed continuously. However, the distribution histograms showed two (phosphorylase and SDHase) or three (acid and alkaline ATPase) clear peaks. Eighteen combinations of reaction intensities (profiles) were seen of which eight were very rare. The distribution of profiles differed between individuals but were similar in right and left muscles.
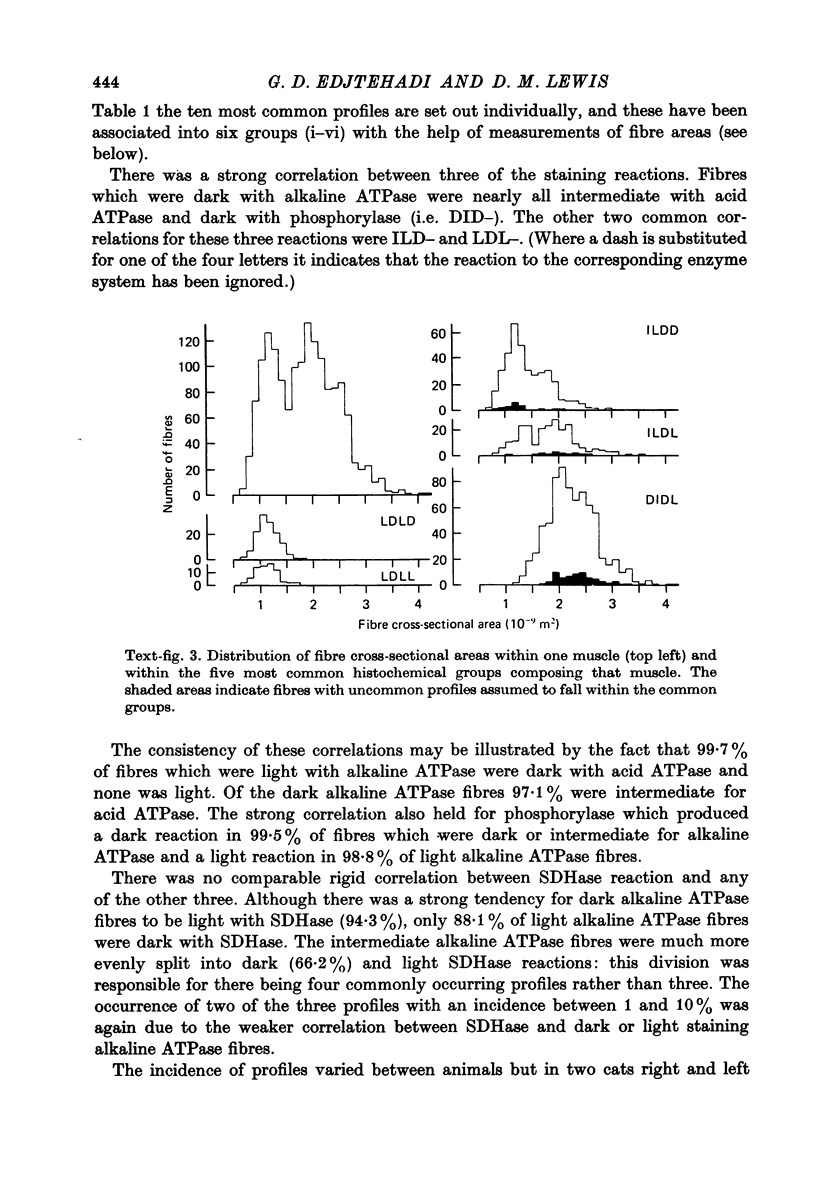
3. Areas of fibres were measured from muscles which had been fixed at the length at which twitch tension was maximal. The variance in fibre area with any one profile was significantly less than the variance in fibre area of all fibres within a muscle. There were significant differences between the mean areas of fibres with different profiles.
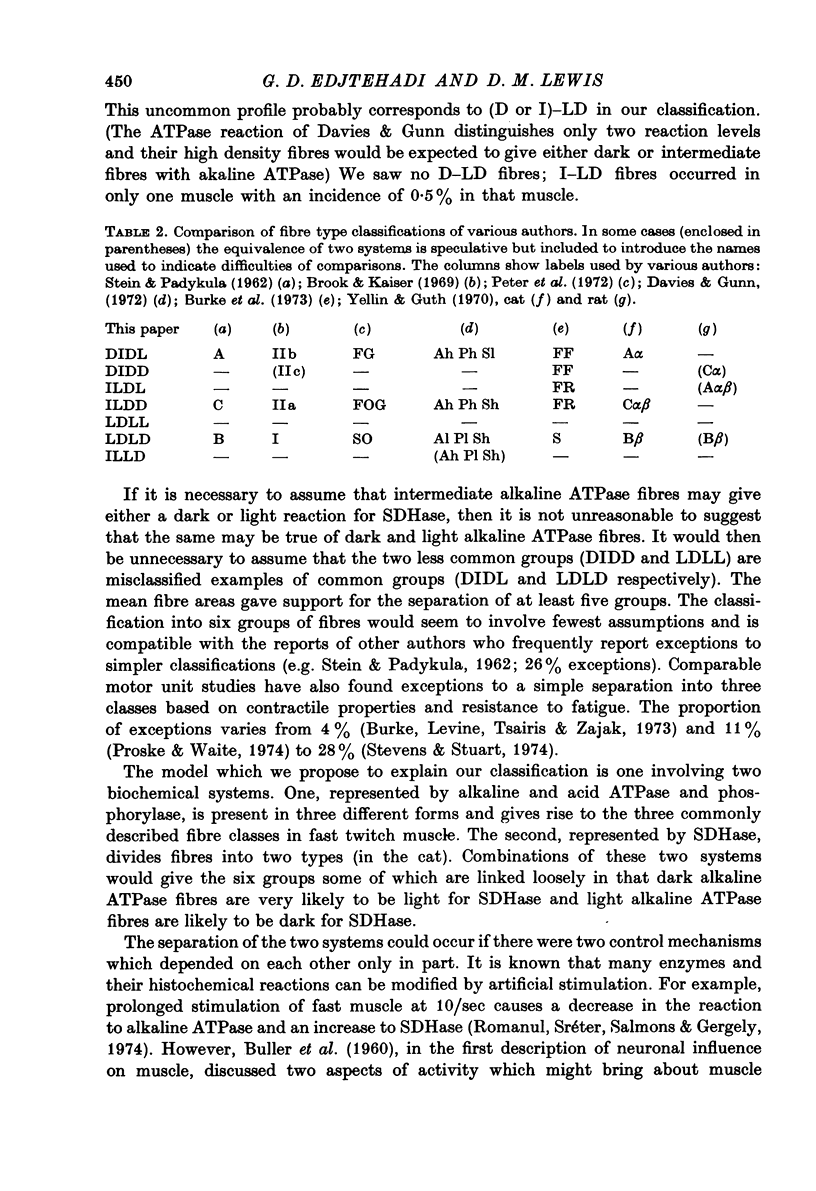
4. If only three enzyme reactions are considered (acid and alkaline ATPase and phosphorylase) the majority of fibres fall into one of the three classes commonly accepted for other muscles. The remainder would fit into this classification with the minimal assumption of only one error of fibre typing resulting from the continuous distributions of enzyme reaction intensities. The SDHase reaction was not strongly correlated with the three classes and could be used to divide the fibres further into six groups. Differences between means of fibre areas were significant for all pairs out of these six groups except one.
5. The grouping may be considered to reflect a dual system of enzymes, the two systems being (a) ATPases and phosphorylase, (b) SDHase. A possible role of nervous activity in determining this dual system is discussed. The hypothesis involves two partly independent characteristics of motoneuronal activity: (a) the frequency of impulses, and (b) the total number of impulses.
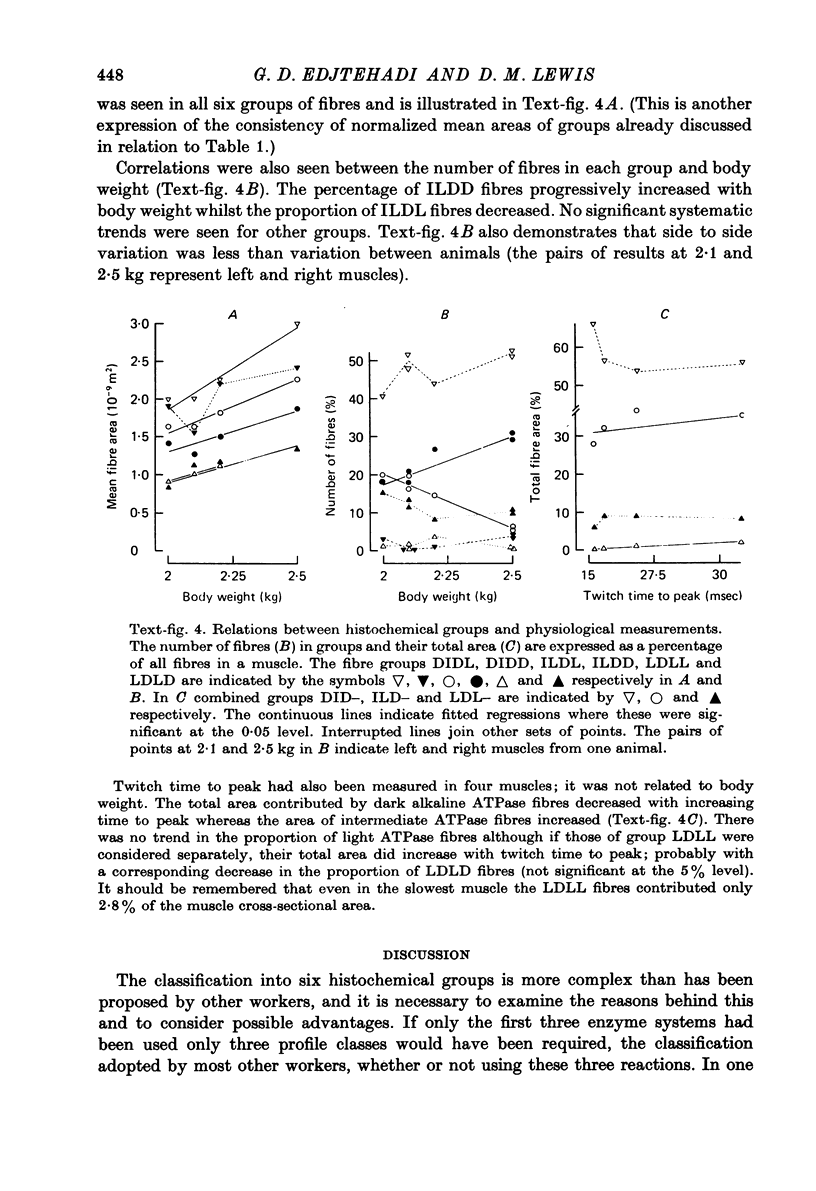
6. The measurements are correlated with other physiological variables in the individual animals. The mean areas of fibres in all groups increased with body weight. There were changes in the proportions of light and dark SDHase fibres related to weight. The total area contributed by dark alkaline ATPase fibres decreased and that by intermediate alkaline ATPase fibres increased with increasing twitch time to peak.
7. Specific tension of the group of slower muscle fibres in f.d.l. was estimated to be 0·29 N.mm-2 compared with 0·39 N.mm-2 for the faster fibres.
Full text
PDF









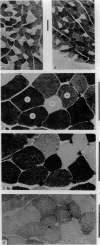
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Knott S., Lewis D. M., Luck J. C., Westerman R. A. Isometric contractions of motor units in a fast twitch muscle of the cat. J Physiol. 1973 May;231(1):87–104. doi: 10.1113/jphysiol.1973.sp010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Some comments on the histochemical characterization of muscle adenosine triphosphatase. J Histochem Cytochem. 1969 Jun;17(6):431–432. doi: 10.1177/17.6.431. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Trophic functions of the neuron. II. Denervation and regulation of muscle. The correlation of physiological properties with histochemical characteristics in single muscle units. Ann N Y Acad Sci. 1974 Mar 22;228(0):145–159. doi: 10.1111/j.1749-6632.1974.tb20507.x. [DOI] [PubMed] [Google Scholar]
- DUBOWITZ V., PEARSE A. G. A comparative histochemical study of oxidative enzyme and phosphorylase activity in skeletal muscle. Z Zellforch Microsk Anat Histochem. 1960;2:105–117. doi: 10.1007/BF00744575. [DOI] [PubMed] [Google Scholar]
- Davies A. S., Gunn H. M. Histochemical fibre types in the mammalian diaphragm. J Anat. 1972 May;112(Pt 1):41–60. [PMC free article] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- Kean C. J., Lewis D. M., McGarrick J. D. Dynamic properties of denervated fast and slow twitch muscle of the cat. J Physiol. 1974 Feb;237(1):103–113. doi: 10.1113/jphysiol.1974.sp010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINE S. W., GLENNER G. G. ULTRARAPID TISSUE FREEZING IN LIQUID NITROGEN. J Histochem Cytochem. 1964 Oct;12:777–783. doi: 10.1177/12.10.777. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Prewitt M. A., Salafsky B. Enzymic and histochemical changes in fast and slow muscles after cross innervation. Am J Physiol. 1970 Jan;218(1):69–74. doi: 10.1152/ajplegacy.1970.218.1.69. [DOI] [PubMed] [Google Scholar]
- Proske U., Waite P. M. Properties of types of motor units in the medial gastrochemius muscle of the cat. Brain Res. 1974 Feb 15;67(1):89–101. doi: 10.1016/0006-8993(74)90300-x. [DOI] [PubMed] [Google Scholar]
- STEIN J. M., PADYKULA H. A. Histochemical classification of individual skeletal muscle fibers of the rat. Am J Anat. 1962 Mar;110:103–123. doi: 10.1002/aja.1001100203. [DOI] [PubMed] [Google Scholar]
- Spurway N. C. Objective typing of mouse ankle-extensor muscle fibres based on histochemical photometry [proceedings]. J Physiol. 1978 Apr;277:47P–48P. [PubMed] [Google Scholar]
- Stephens J. A., Stuart D. G. Proceedings: The classification of motor units in cat medial gastrocnemius muscle. J Physiol. 1974 Jul;240(2):43P–44P. [PubMed] [Google Scholar]
- Yellin H., Guth L. The histochemical classification of muscle fibers. Exp Neurol. 1970 Feb;26(2):424–432. doi: 10.1016/0014-4886(70)90137-8. [DOI] [PubMed] [Google Scholar]
- al-Amood W. S., Pope R. A comparison of the structural features of muscle fibres from a fast- and a slow-twitch muscle of the pelvic limb of the cat. J Anat. 1972 Oct;113(Pt 1):49–60. [PMC free article] [PubMed] [Google Scholar]