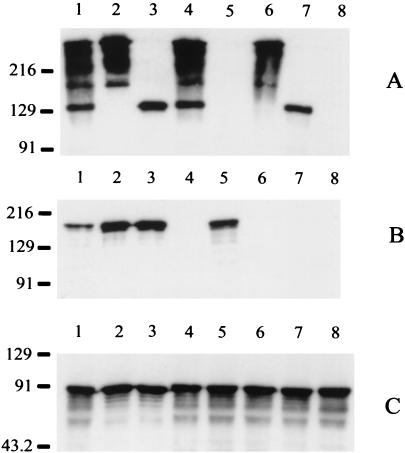
FIG. 1.
Expression of Hag, UspA1, and UspA2 by wild-type and mutant strains of M. catarrhalis O35E. Whole-cell lysates were probed in Western blot analysis with UspA1- and UspA2-reactive MAb 17C7 (A), with Hag-specific MAb 5D2 (B), and with CopB-specific MAb 10F3 (C). Lanes: 1, wild-type parent strain; 2, uspA1 mutant O35E.118CAT; 3, uspA2 mutant O35E.2ZEO; 4, hag mutant O35E.HG; 5, uspA1 uspA2 double mutant O35E.ZC; 6, uspA1 hag double mutant O35E.1HG; 7, uspA2 hag double mutant O35E.2HG; 8, uspA1 uspA2 hag triple mutant O35E.ZCS. In this gel system, as described previously (13), the UspA1 protein migrates as a 130-kDa band whereas the UspA2 protein migrates as a series of bands near the top of the gel. Both UspA1 and UspA2 bind MAb 17C7. The CopB protein was used as an internal control for standardizing antigen loads. Molecular size position markers (in kilodaltons) are shown on the left.