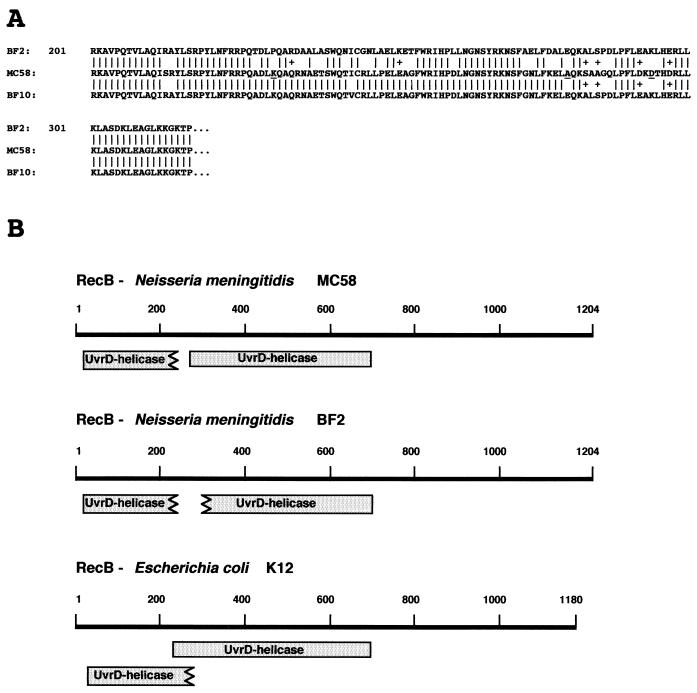
FIG. 4.
Partial primary-structure and functional domains of the RecB proteins from BF2, MC58, and BF10. (A) Alignment of deduced partial amino acid sequences of the RecB proteins from BF2, MC58, and BF10. Vertical bars indicate identical amino acids; pluses indicate synonymous substitutions. Amino acids that are evolutionarily conserved in the RecB proteins from many bacteria are underlined in the RecB sequences of MC58. (B) Location of UvrD-like type II helicase domains in RecB proteins from N. meningitidis strains MC58 and BF2 and from E. coli K-12. Domains were identified by computer program analysis (CDD at the National Center for Biotechnology Information [http://www.ncbi.nlm.nih.gov:80/Structure/cdd/cdd.shtml]) designed to identify the conserved domains present in a protein sequence (shaded boxes). The lengths of the RecB proteins from MC58 and BF2 are identical (1,204 amino acids); E. coli K-12 RecB has 1,180 residues.