Abstract
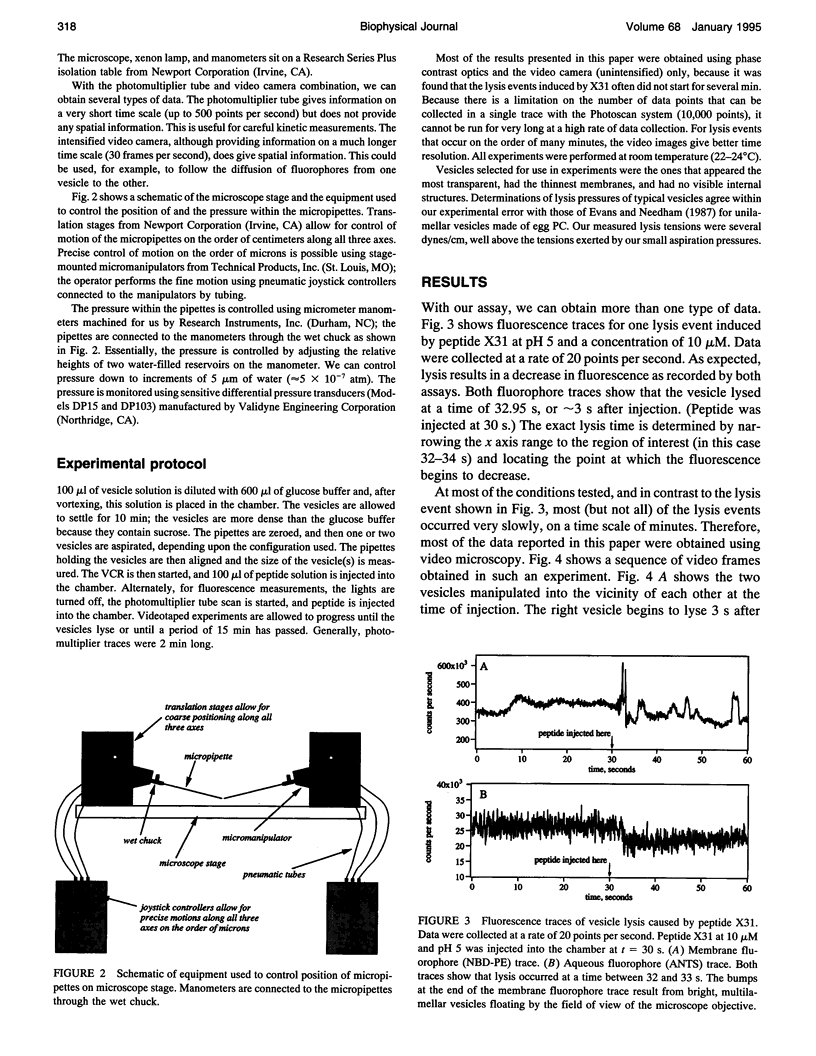
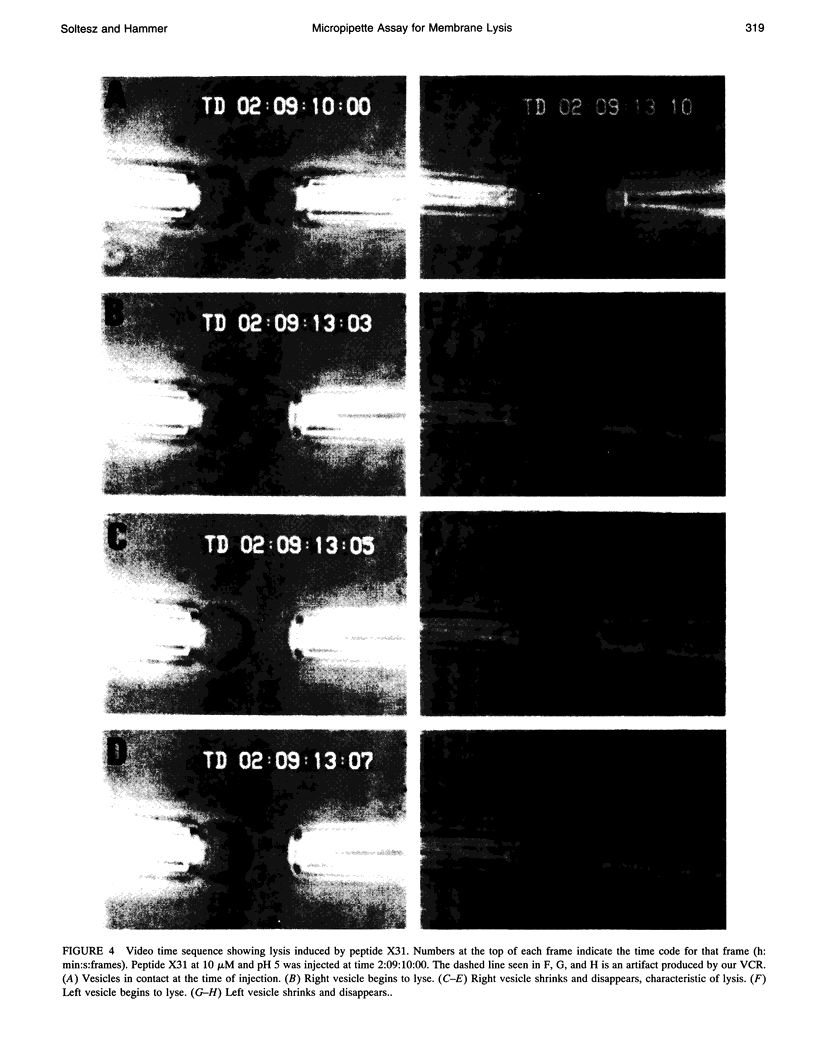
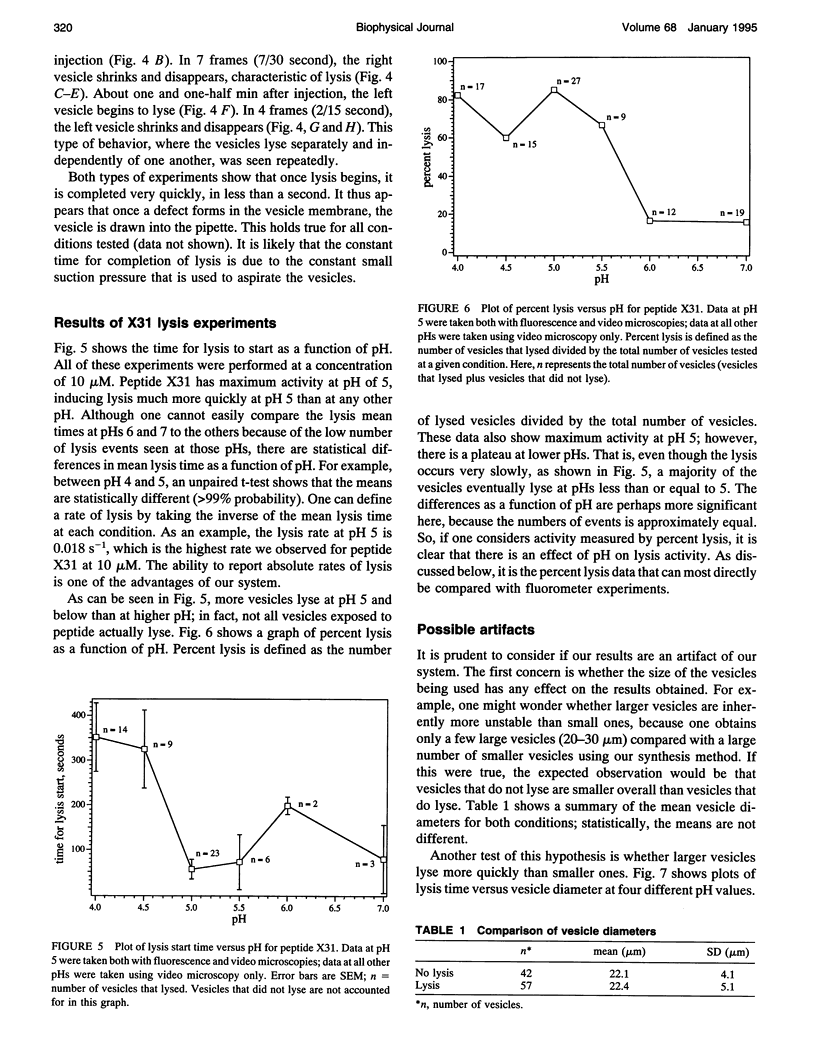
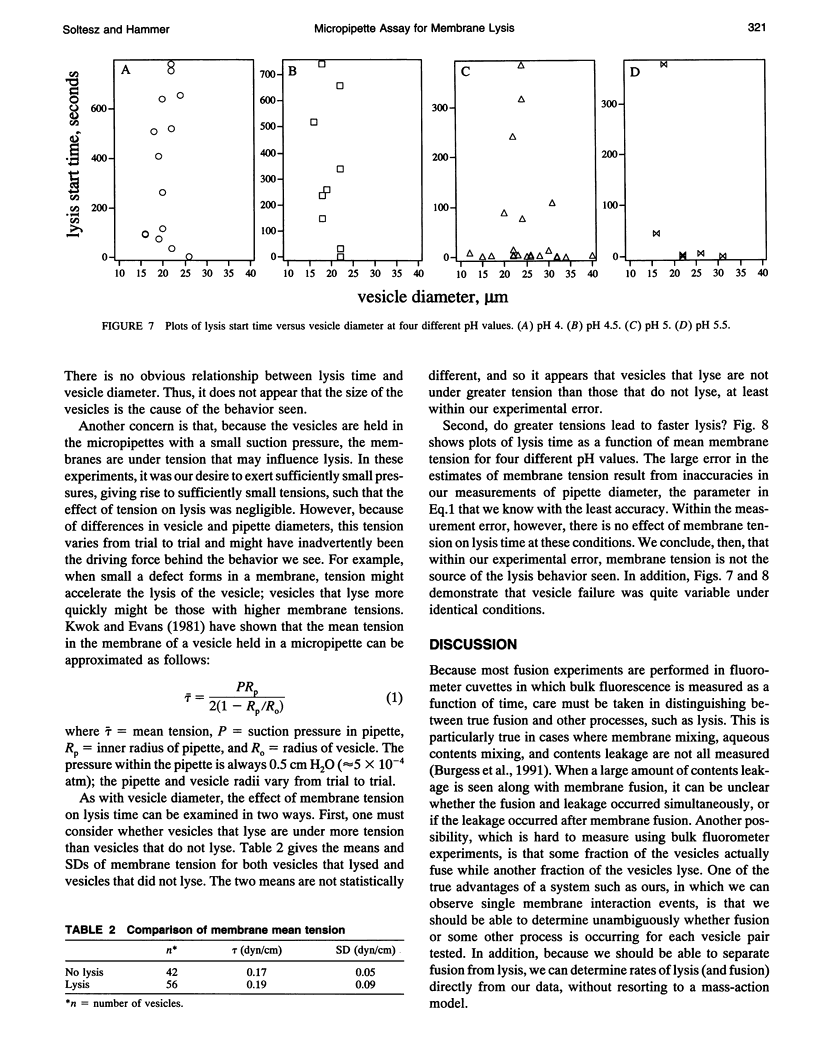
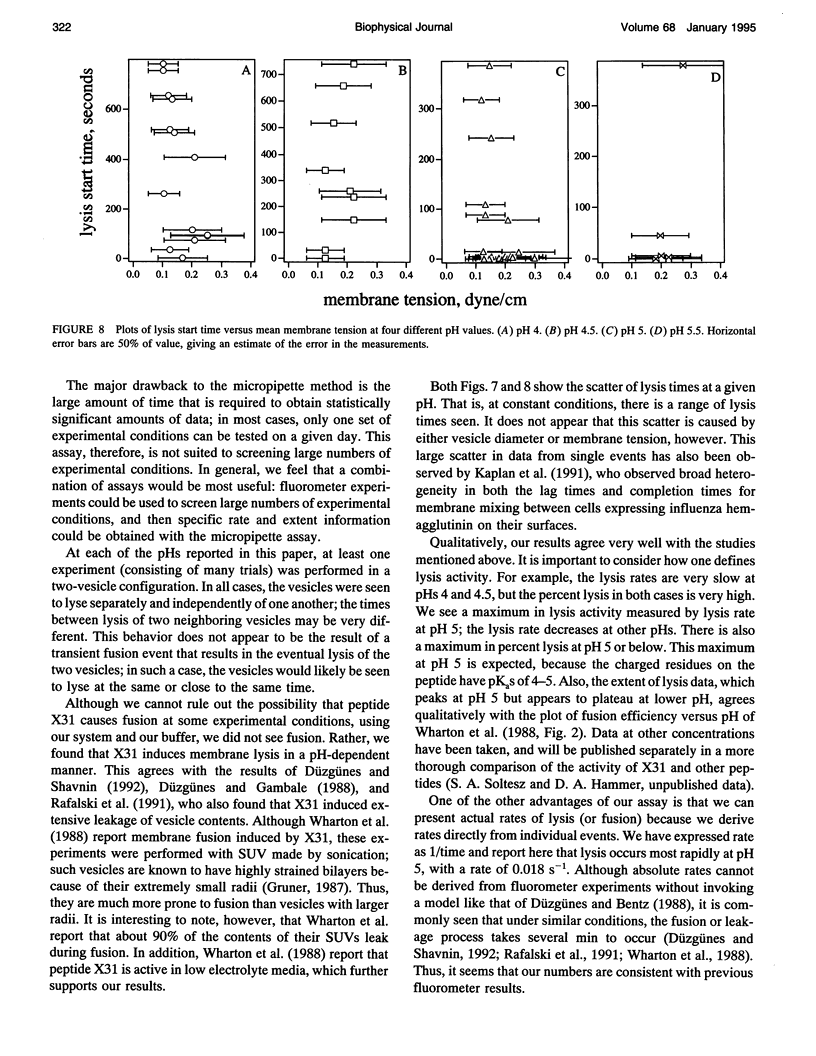
We have assembled a micropipette aspiration assay to measure membrane destabilization events in which large (20-30 microns diameter) unilamellar vesicles are manipulated and exposed to membrane destabilizing agents. Single events can be seen with a light microscope and are recorded using both a video camera and a photomultiplier tube. We have performed experiments with a wild-type fusion peptide from influenza virus (X31) and found that it induces pH-dependent, stochastic lysis of large unilamellar vesicles. The rate and extent of lysis are both maximum at pH 5; the maximum rate of lysis is 0.018 s-1 at pH 5. An analysis of our data indicates that the lysis is not correlated either to the size of the vesicles or to the tension created in the vesicle membranes by aspiration.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentz J., Ellens H., Alford D. An architecture for the fusion site of influenza hemagglutinin. FEBS Lett. 1990 Dec 10;276(1-2):1–5. doi: 10.1016/0014-5793(90)80492-2. [DOI] [PubMed] [Google Scholar]
- Burgess S. W., Massenburg D., Yates J., Lentz B. R. Poly(ethylene glycol)-induced lipid mixing but not fusion between synthetic phosphatidylcholine large unilamellar vesicles. Biochemistry. 1991 Apr 30;30(17):4193–4200. doi: 10.1021/bi00231a013. [DOI] [PubMed] [Google Scholar]
- Carr C. M., Kim P. S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993 May 21;73(4):823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Daniels R. S., Downie J. C., Hay A. J., Knossow M., Skehel J. J., Wang M. L., Wiley D. C. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell. 1985 Feb;40(2):431–439. doi: 10.1016/0092-8674(85)90157-6. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Gambale F. Membrane action of synthetic N-terminal peptides of influenza virus hemagglutinin and its mutants. FEBS Lett. 1988 Jan 25;227(2):110–114. doi: 10.1016/0014-5793(88)80879-2. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Shavnin S. A. Membrane destabilization by N-terminal peptides of viral envelope proteins. J Membr Biol. 1992 May;128(1):71–80. doi: 10.1007/BF00231872. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Minimum energy analysis of membrane deformation applied to pipet aspiration and surface adhesion of red blood cells. Biophys J. 1980 May;30(2):265–284. doi: 10.1016/S0006-3495(80)85093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Metcalfe M. Free energy potential for aggregation of giant, neutral lipid bilayer vesicles by Van der Waals attraction. Biophys J. 1984 Sep;46(3):423–426. doi: 10.1016/S0006-3495(84)84039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Metcalfe M. Free energy potential for aggregation of mixed phosphatidylcholine/phosphatidylserine lipid vesicles in glucose polymer (dextran) solutions. Biophys J. 1984 Apr;45(4):715–720. doi: 10.1016/S0006-3495(84)84213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Doms R. W., York D., White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986 Jan;102(1):11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy H. R., Durell S. R., Schoch C., Blumenthal R. Analyzing the fusion process of influenza hemagglutinin by mutagenesis and molecular modeling. Biophys J. 1992 Apr;62(1):95–97. doi: 10.1016/S0006-3495(92)81790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D. Membrane fusion of enveloped viruses: especially a matter of proteins. J Bioenerg Biomembr. 1990 Apr;22(2):121–155. doi: 10.1007/BF00762943. [DOI] [PubMed] [Google Scholar]
- Kaplan D., Zimmerberg J., Puri A., Sarkar D. P., Blumenthal R. Single cell fusion events induced by influenza hemagglutinin: studies with rapid-flow, quantitative fluorescence microscopy. Exp Cell Res. 1991 Jul;195(1):137–144. doi: 10.1016/0014-4827(91)90509-s. [DOI] [PubMed] [Google Scholar]
- Kemble G. W., Danieli T., White J. M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994 Jan 28;76(2):383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M. Time-resolved capacitance measurements: monitoring exocytosis in single cells. Q Rev Biophys. 1991 Feb;24(1):75–101. doi: 10.1017/s0033583500003279. [DOI] [PubMed] [Google Scholar]
- Liu Z. Y., Solow R., Hu V. W. Fluorescence analysis of size distribution and mode of dye release from carboxyfluorescein-loaded vesicles: application to the study of complement-membrane interactions. Biochim Biophys Acta. 1988 Nov 22;945(2):253–262. doi: 10.1016/0005-2736(88)90487-7. [DOI] [PubMed] [Google Scholar]
- Marra J., Israelachvili J. Direct measurements of forces between phosphatidylcholine and phosphatidylethanolamine bilayers in aqueous electrolyte solutions. Biochemistry. 1985 Aug 13;24(17):4608–4618. doi: 10.1021/bi00338a020. [DOI] [PubMed] [Google Scholar]
- Melikyan G. B., Niles W. D., Peeples M. E., Cohen F. S. Influenza hemagglutinin-mediated fusion pores connecting cells to planar membranes: flickering to final expansion. J Gen Physiol. 1993 Dec;102(6):1131–1149. doi: 10.1085/jgp.102.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D., Evans E. Structure and mechanical properties of giant lipid (DMPC) vesicle bilayers from 20 degrees C below to 10 degrees C above the liquid crystal-crystalline phase transition at 24 degrees C. Biochemistry. 1988 Oct 18;27(21):8261–8269. doi: 10.1021/bi00421a041. [DOI] [PubMed] [Google Scholar]
- Needham D. Measurement of interbilayer adhesion energies. Methods Enzymol. 1993;220:111–129. doi: 10.1016/0076-6879(93)20078-h. [DOI] [PubMed] [Google Scholar]
- Niles W. D., Cohen F. S. Video fluorescence microscopy studies of phospholipid vesicle fusion with a planar phospholipid membrane. Nature of membrane-membrane interactions and detection of release of contents. J Gen Physiol. 1987 Nov;90(5):703–735. doi: 10.1085/jgp.90.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir S., Andersen M. Van der Waals interactions between cell surfaces. J Membr Biol. 1977 Feb 24;31(1-2):1–18. doi: 10.1007/BF01869396. [DOI] [PubMed] [Google Scholar]
- Perin M. S., MacDonald R. C. Fusion of synaptic vesicle membranes with planar bilayer membranes. Biophys J. 1989 May;55(5):973–986. doi: 10.1016/S0006-3495(89)82896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski M., Ortiz A., Rockwell A., van Ginkel L. C., Lear J. D., DeGrado W. F., Wilschut J. Membrane fusion activity of the influenza virus hemagglutinin: interaction of HA2 N-terminal peptides with phospholipid vesicles. Biochemistry. 1991 Oct 22;30(42):10211–10220. doi: 10.1021/bi00106a020. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Schoch C., Blumenthal R. Role of the fusion peptide sequence in initial stages of influenza hemagglutinin-induced cell fusion. J Biol Chem. 1993 May 5;268(13):9267–9274. [PubMed] [Google Scholar]
- Simon S. A., Disalvo E. A., Gawrisch K., Borovyagin V., Toone E., Schiffman S. S., Needham D., McIntosh T. J. Increased adhesion between neutral lipid bilayers: interbilayer bridges formed by tannic acid. Biophys J. 1994 Jun;66(6):1943–1958. doi: 10.1016/S0006-3495(94)80988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., Doms R. W., Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Stegmann T., White J. M., Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990 Dec;9(13):4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancrède P., Paquin P., Houle A., Leblanc R. M. Formation of asymmetrical planar lipid bilayer membranes from characterized monolayers. J Biochem Biophys Methods. 1983 Jul;7(4):299–310. doi: 10.1016/0165-022x(83)90055-6. [DOI] [PubMed] [Google Scholar]
- Tozeren A., Sung K. L., Chien S. Theoretical and experimental studies on cross-bridge migration during cell disaggregation. Biophys J. 1989 Mar;55(3):479–487. doi: 10.1016/S0006-3495(89)82841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse F. W., Iwata A., Almers W. Membrane flux through the pore formed by a fusogenic viral envelope protein during cell fusion. J Cell Biol. 1993 May;121(3):543–552. doi: 10.1083/jcb.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton S. A., Martin S. R., Ruigrok R. W., Skehel J. J., Wiley D. C. Membrane fusion by peptide analogues of influenza virus haemagglutinin. J Gen Virol. 1988 Aug;69(Pt 8):1847–1857. doi: 10.1099/0022-1317-69-8-1847. [DOI] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
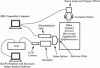
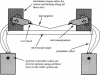
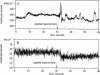
- White S. H. Formation of "solvent-free" black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys J. 1978 Sep;23(3):337–347. doi: 10.1016/S0006-3495(78)85453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D. J., Hall J. E. Role of channels in the fusion of vesicles with a planar bilayer. Biophys J. 1988 Dec;54(6):1053–1063. doi: 10.1016/S0006-3495(88)83042-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Taylor A., Yellen A., Caton A., Gerhard W., Bächi T. Mutations in or near the fusion peptide of the influenza virus hemagglutinin affect an antigenic site in the globular region. J Virol. 1993 Feb;67(2):933–942. doi: 10.1128/jvi.67.2.933-942.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev D. V., Needham D. Tension-stabilized pores in giant vesicles: determination of pore size and pore line tension. Biochim Biophys Acta. 1993 Apr 8;1147(1):89–104. doi: 10.1016/0005-2736(93)90319-u. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Vogel S. S., Chernomordik L. V. Mechanisms of membrane fusion. Annu Rev Biophys Biomol Struct. 1993;22:433–466. doi: 10.1146/annurev.bb.22.060193.002245. [DOI] [PubMed] [Google Scholar]