Abstract
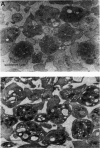
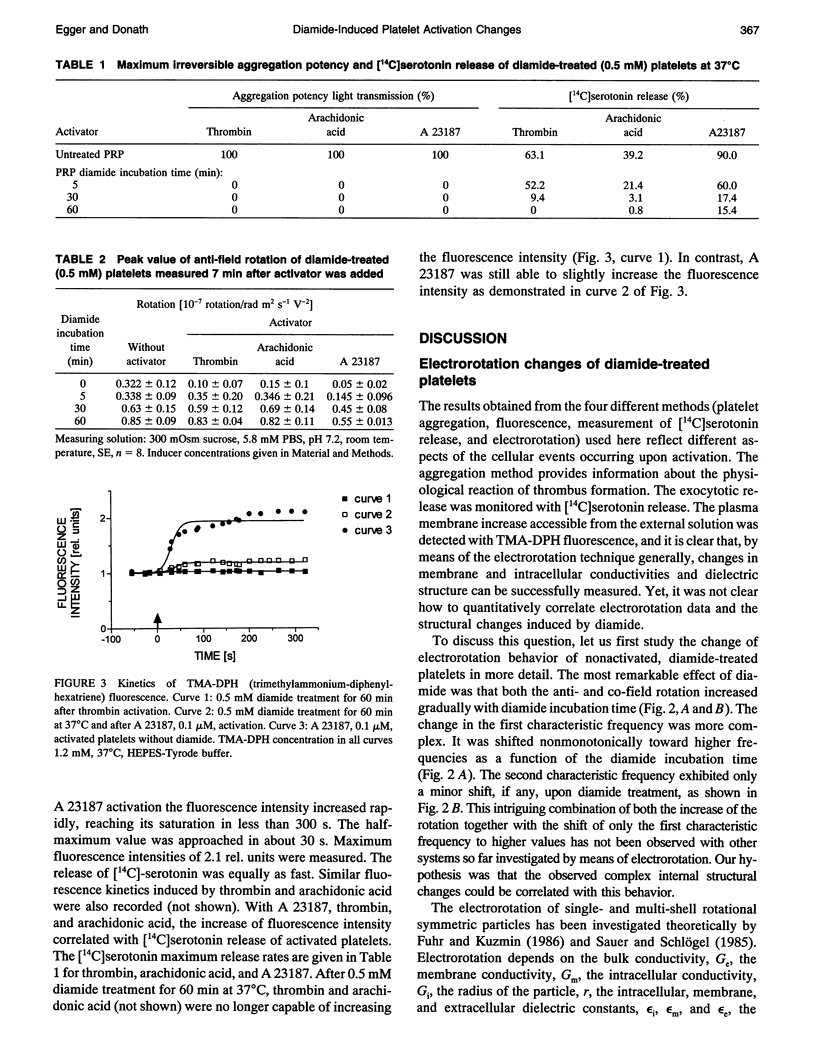
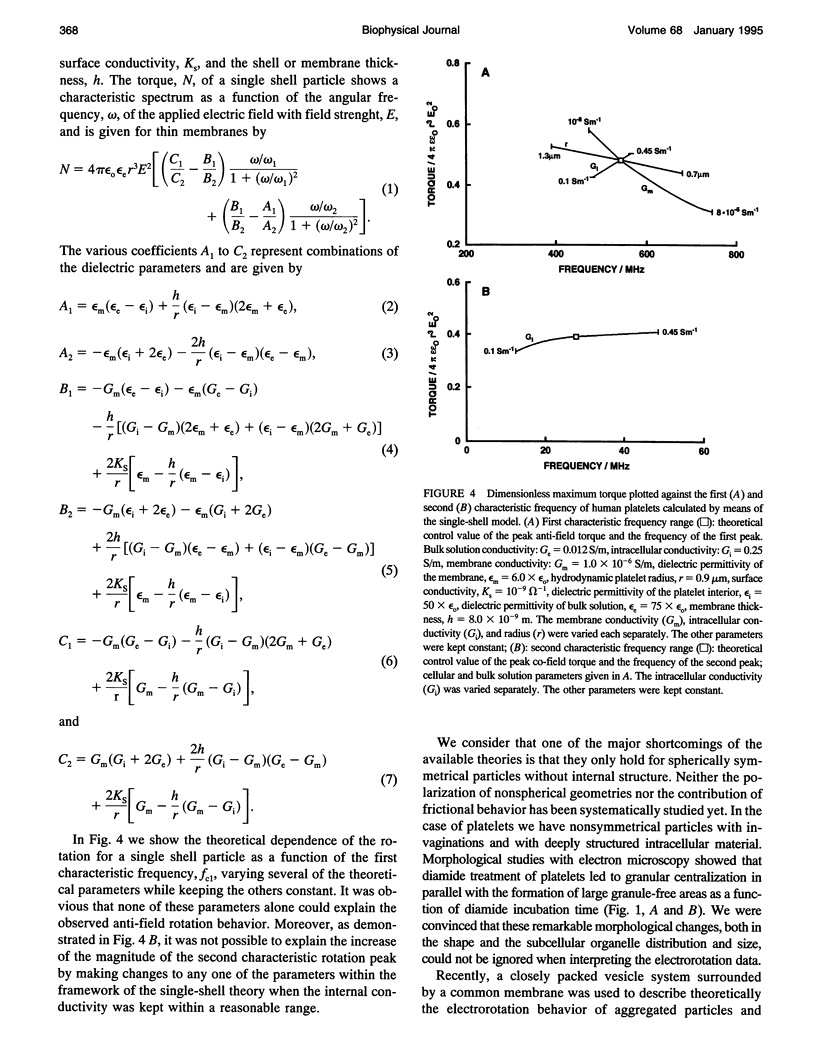
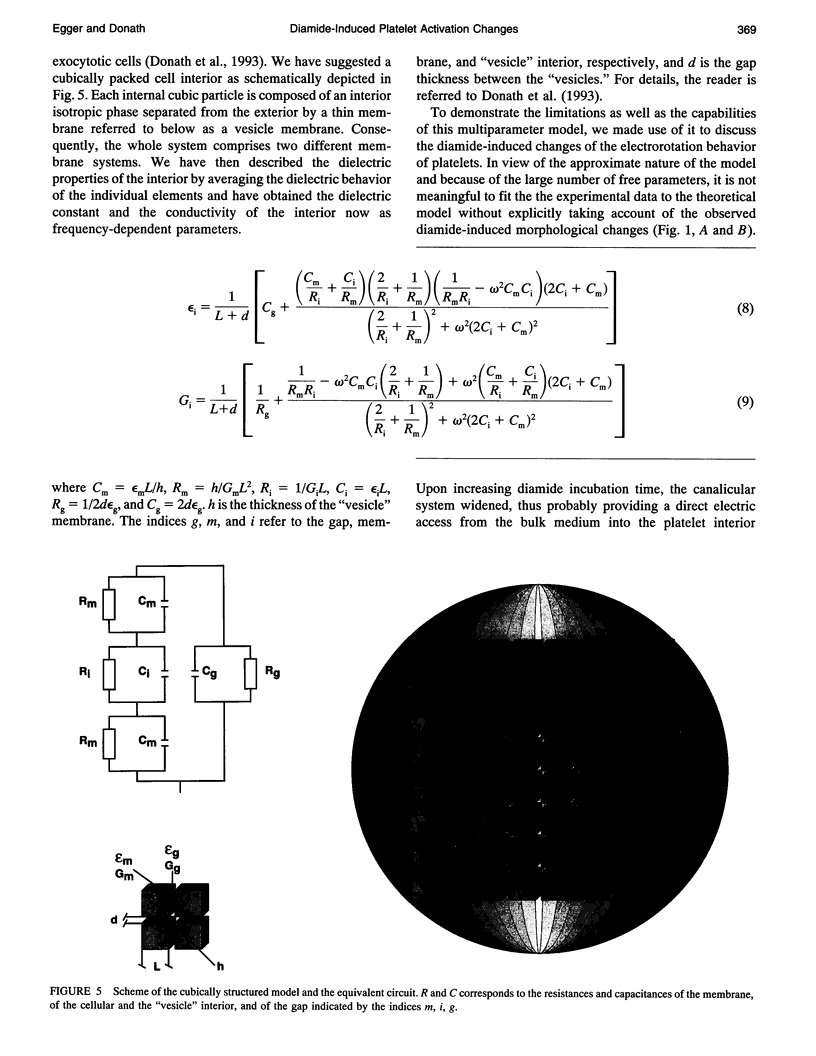
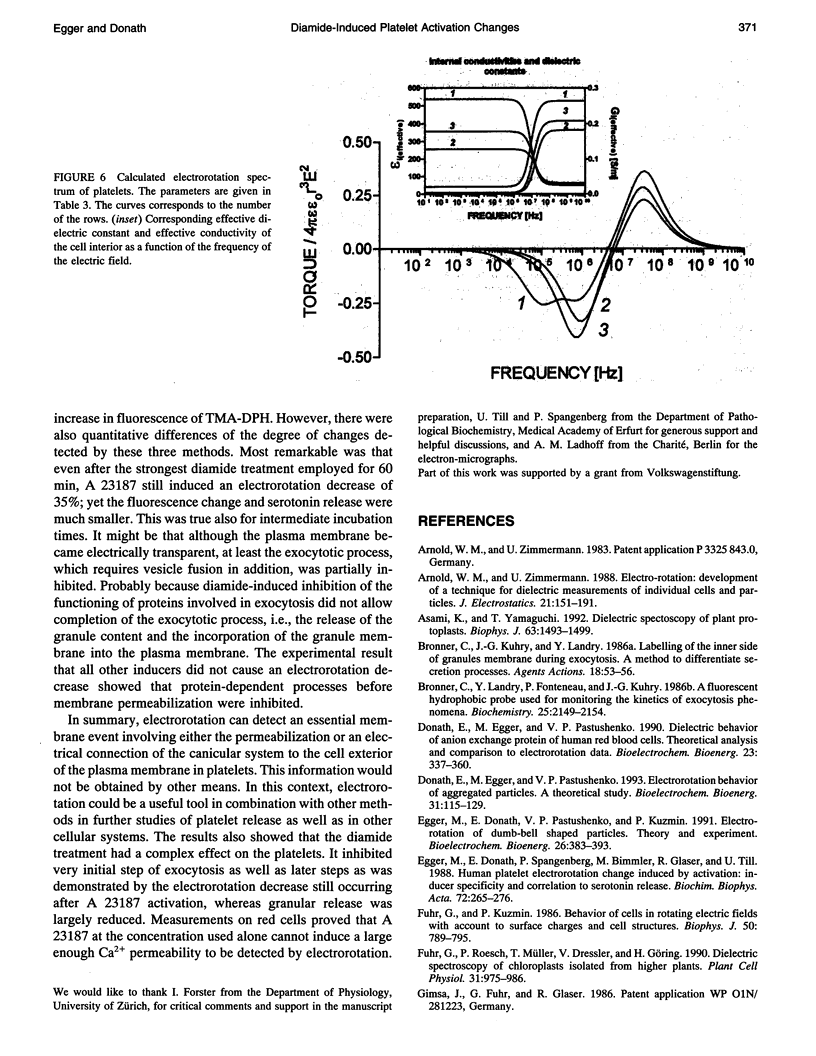
Electrorotation is a special dielectric spectroscopic technique capable of measuring the polarizability of single platelets. The rotational speed of the particles is recorded as a function of the frequency of the applied rotating electric field. As previously shown, the speed of electrorotation in the range of the first characteristic frequency (anti-field rotation) decreased upon activation and was correlated with [14C]serotonin release and an increase of the TMA-DPH-induced fluorescence. Diamide upon activation and was correlated with [14C]serotonin release and an increase of the TMA-DPH-induced fluorescence. Diamide incubation induced morphological changes in control platelets. These changes were accompanied by a shift of the first characteristic frequency of electrorotation toward higher values and a parallel increase of the anti-field rotation. This was explained by a decrease of membrane conductivity and by the changed polarizability of platelet interior due to the observed internal platelet structure changes. Diamide inhibited activation assessed by both electrorotation and TMA-DPH fluorescence in the case of all activators except the ionophore A 23187. Because diamide largely inhibited the A 23187-induced serotonin release, it was concluded that, despite the diamide treatment, the direct increase of cytoplasmic Ca2+ was still able to induce membrane conductivity changes accessible by electrorotation, but this did not complete the final release step of the activation process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami K., Yamaguchi T. Dielectric spectroscopy of plant protoplasts. Biophys J. 1992 Dec;63(6):1493–1499. doi: 10.1016/S0006-3495(92)81734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner C., Kuhry J. G., Landry Y. Labelling of the inner side of granules membrane during exocytosis. A method to differentiate secretion processes. Agents Actions. 1986 Apr;18(1-2):53–56. doi: 10.1007/BF01987981. [DOI] [PubMed] [Google Scholar]
- Bronner C., Landry Y., Fonteneau P., Kuhry J. G. A fluorescent hydrophobic probe used for monitoring the kinetics of exocytosis phenomena. Biochemistry. 1986 Apr 22;25(8):2149–2154. doi: 10.1021/bi00356a045. [DOI] [PubMed] [Google Scholar]
- Egger M., Donath E., Spangenberg P., Bimmler M., Glaser R., Till U. Human platelet electrorotation change induced by activation: inducer specificity and correlation to serotonin release. Biochim Biophys Acta. 1988 Dec 9;972(3):265–276. doi: 10.1016/0167-4889(88)90201-7. [DOI] [PubMed] [Google Scholar]
- Fuhr G., Kuzmin P. I. Behavior of cells in rotating electric fields with account to surface charges and cell structures. Biophys J. 1986 Nov;50(5):789–795. doi: 10.1016/S0006-3495(86)83519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa J., Schnelle T., Zechel G., Glaser R. Dielectric spectroscopy of human erythrocytes: investigations under the influence of nystatin. Biophys J. 1994 Apr;66(4):1244–1253. doi: 10.1016/S0006-3495(94)80908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S., Bevan J., Cockbill S. R., Hanley S. P., Parry M. J. Effects of a selective inhibitor of thromboxane synthetase on human blood platelet behaviour. Thromb Res. 1980 Oct 15;20(2):219–230. doi: 10.1016/0049-3848(80)90387-4. [DOI] [PubMed] [Google Scholar]
- Hill T. D., White J. G., Rao G. H. The influence of glutathione depleting agents on human platelet function. Thromb Res. 1989 Mar 1;53(5):457–465. doi: 10.1016/0049-3848(89)90200-4. [DOI] [PubMed] [Google Scholar]
- Hofmann B., Danz R., Hofmann J., Pescarmona G., Bosia A., Meyer M., Lösche W., Till U. Differences in morphology and protein pattern of human blood platelets during irreversible and diamide mediated reversible aggregation. Biomed Biochim Acta. 1983;42(5):489–501. [PubMed] [Google Scholar]
- Kitajima H., Yamaguchi T., Kimoto E. Hemolysis of human erythrocytes under hydrostatic pressure is suppressed by cross-linking of membrane proteins. J Biochem. 1990 Dec;108(6):1057–1062. doi: 10.1093/oxfordjournals.jbchem.a123305. [DOI] [PubMed] [Google Scholar]
- Klonk S., Deuticke B. Involvement of cytoskeletal proteins in the barrier function of the human erythrocyte membrane. I. Impairment of resealing and formation of aqueous pores in the ghost membrane after modification of SH groups. Biochim Biophys Acta. 1992 Apr 29;1106(1):126–136. doi: 10.1016/0005-2736(92)90230-j. [DOI] [PubMed] [Google Scholar]
- Spangenberg P., Till U., Gschmeissner S., Crawford N. Changes in the distribution and organization of platelet actin induced by diamide and its functional consequences. Br J Haematol. 1987 Dec;67(4):443–450. doi: 10.1111/j.1365-2141.1987.tb06167.x. [DOI] [PubMed] [Google Scholar]
- Wu X. B., Brüne B., von Appen F., Ullrich V. Reversible activation of soluble guanylate cyclase by oxidizing agents. Arch Biochem Biophys. 1992 Apr;294(1):75–82. doi: 10.1016/0003-9861(92)90139-n. [DOI] [PubMed] [Google Scholar]