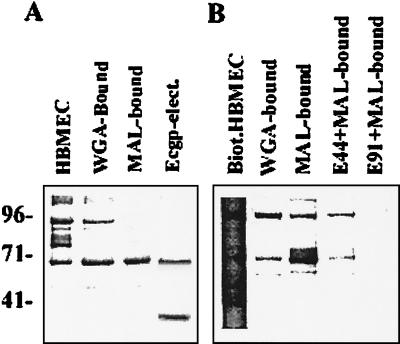
FIG. 2.
Identification of OmpA-bound proteins from MAL affinity chromatography-purified proteins. HBMEC membrane proteins either unbiotinylated or biotinylated were initially subjected to WGA affinity chromatography, and the WGA-bound proteins were further subjected to MAL chromatography. The lectin-bound protein fractions were separated by SDS-10%PAGE. The unbiotinylated proteins were stained with Coomassie brilliant blue (A), and the biotinylated proteins were transferred to nitrocellulose, followed by probing with streptavidin-peroxidase (B). In addition, the 95-kDa protein from the unbiotinylated MAL-bound fraction was electroeluted, frozen at −20°C overnight, thawed, and subjected to SDS-PAGE (Ecgp-elect.).