Abstract
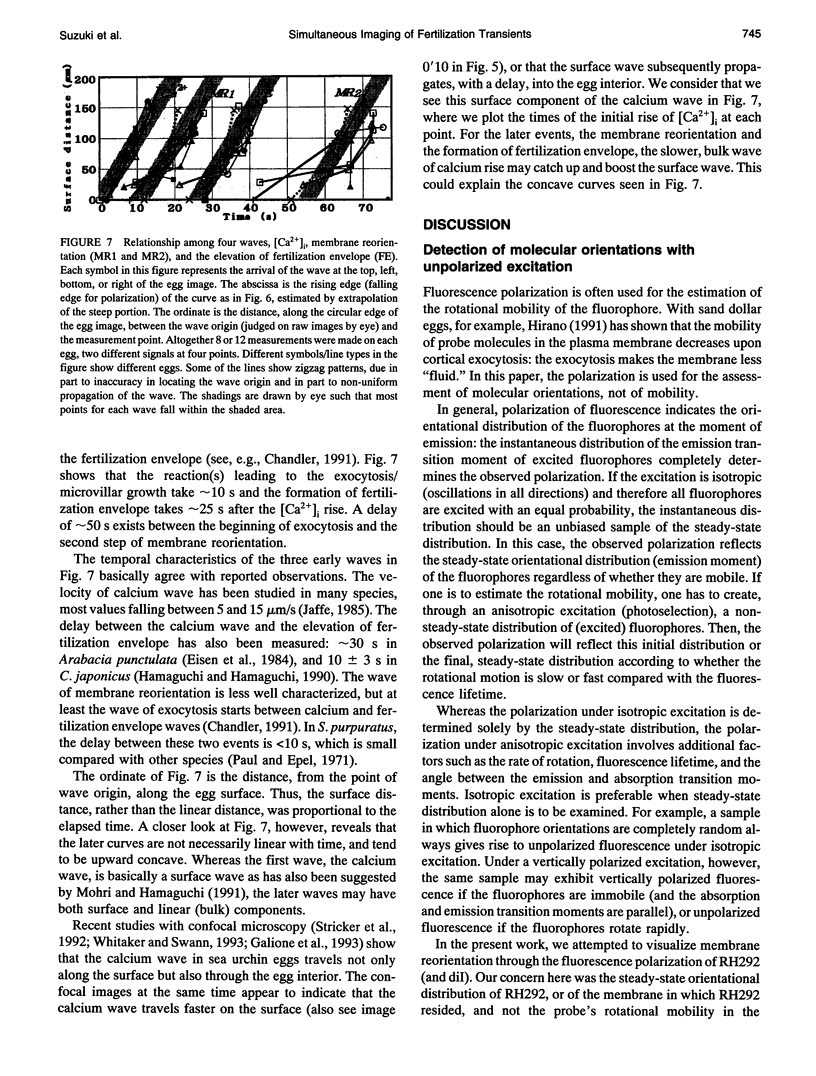
Four early events of egg fertilization, changes in intracellular calcium concentration and intracellular pH, reorientation of the surface membrane, and the elevation of the fertilization envelope, were imaged in real time and in pairs in single sea urchin eggs. The paired imaging allowed the correlation of the four events spatially and temporally. Three of them propagated as waves starting at the sperm entry site. The earliest was the calcium wave, visualized with fluorescent indicator dyes. After a delay of 10 s there followed a large decrease in the fluorescence polarization of membrane-bound dyes, which we interpret as arising from membrane reorientation as a result of cortical granule exocytosis and microvillar elongation. With a further delay of 15 s the fertilization envelope was seen to rise in transmitted light. All three waves propagated with similar velocities of approximately 10 microns/s, supporting the view that calcium triggers the latter two events. The fluorescence polarization changed in two steps with a clear pause of 10-20 s in between. The second step, which also propagated as wave, reflects either further elongation of microvilli or straightening of irregular microvilli. This second step was abolished by cytochalasin B and was coincident with an increase in cytoplasmic pH, suggesting that pH-induced actin reorganization may play a role. The cytoplasmic alkalinization, imaged with a fluorescent probe, was quite different from the other events in that it took place homogeneously throughout the egg and slowly (over 100 s). Apparently, the alkalinization is not on a direct downstream pathway of calcium origin. An opposing possibility, that the alkalinization may in fact be triggered by the traveling calcium wave, is also discussed.
Full text
PDF

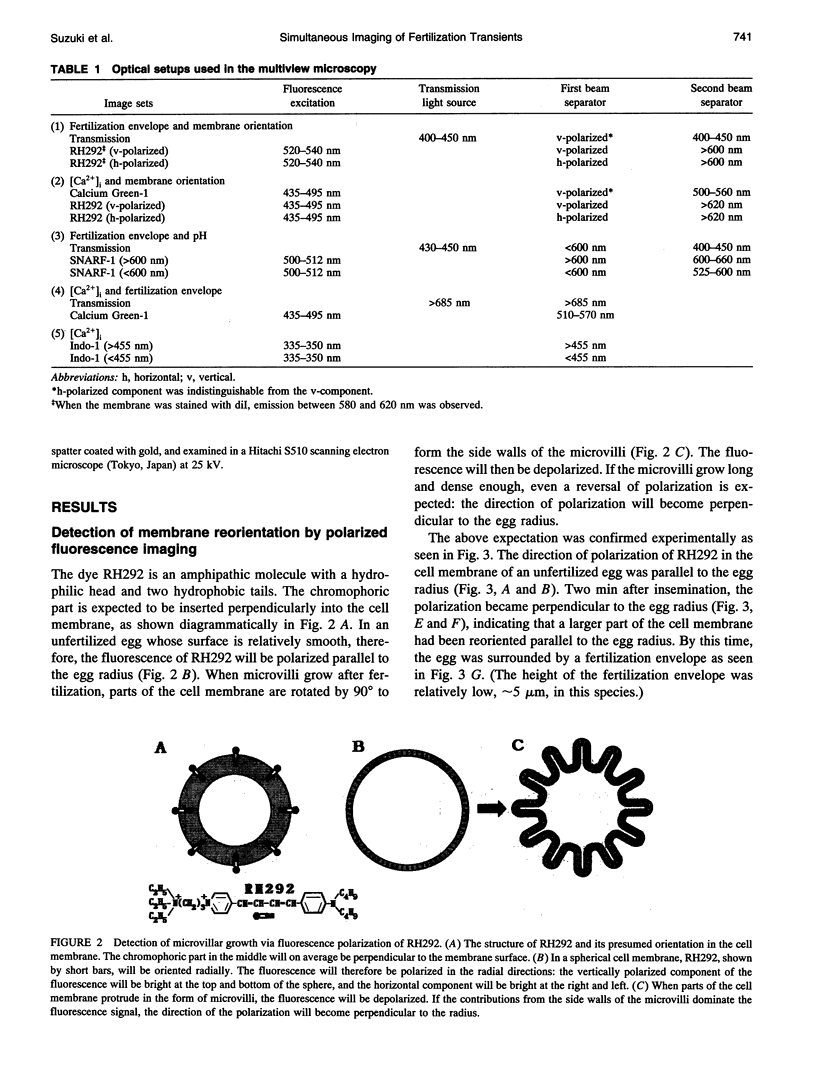

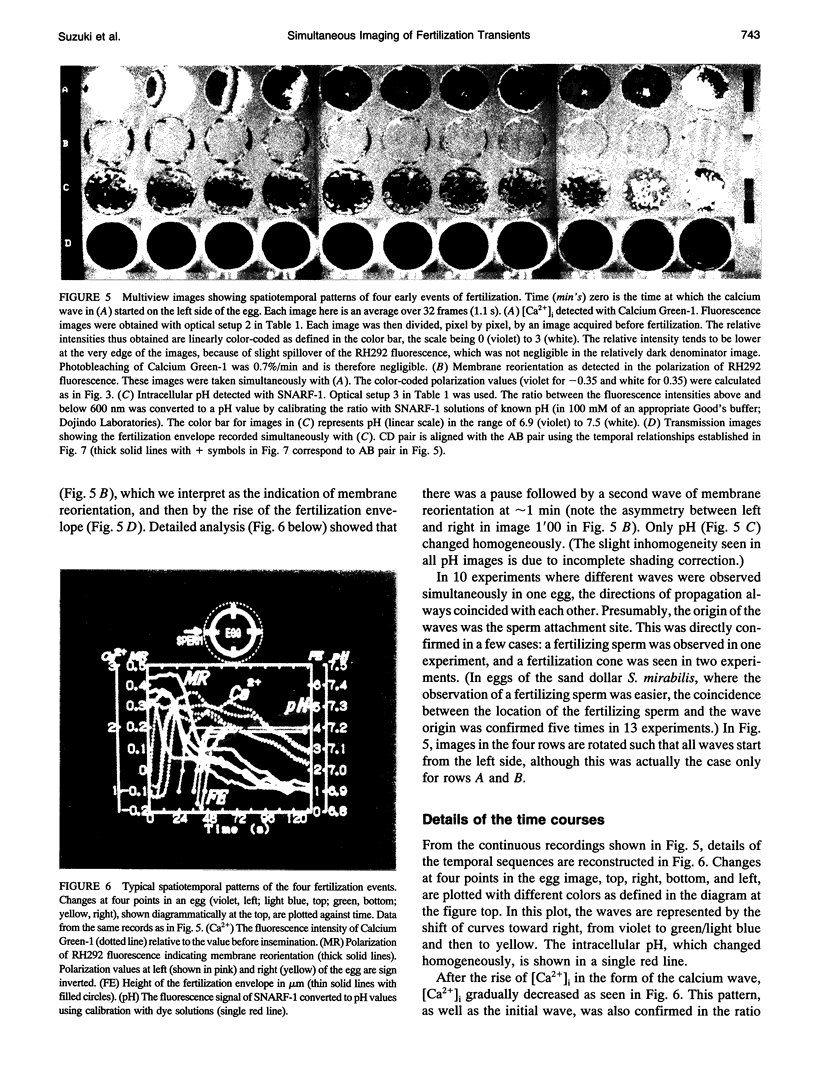




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. Carbocyanine dye orientation in red cell membrane studied by microscopic fluorescence polarization. Biophys J. 1979 Jun;26(3):557–573. doi: 10.1016/S0006-3495(79)85271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg D. A., Rebhun L. I., Hyatt H. Structural organization of actin in the sea urchin egg cortex: microvillar elongation in the absence of actin filament bundle formation. J Cell Biol. 1982 Apr;93(1):24–32. doi: 10.1083/jcb.93.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Chambers E. L., de Armendi J. Membrane potential, action potential and activation potential of eggs of the sea urchin, Lytechinus variegatus. Exp Cell Res. 1979 Aug;122(1):203–218. doi: 10.1016/0014-4827(79)90575-5. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Heuser J. Membrane fusion during secretion: cortical granule exocytosis in sex urchin eggs as studied by quick-freezing and freeze-fracture. J Cell Biol. 1979 Oct;83(1):91–108. doi: 10.1083/jcb.83.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. E. Multiple intracellular signals coordinate structural dynamics in the sea urchin egg cortex at fertilization. J Electron Microsc Tech. 1991 Mar;17(3):266–293. doi: 10.1002/jemt.1060170304. [DOI] [PubMed] [Google Scholar]
- Eddy E. M., Shapiro B. M. Changes in the topography of the sea urchin egg after fertilization. J Cell Biol. 1976 Oct;71(1):35–48. doi: 10.1083/jcb.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A., Kiehart D. P., Wieland S. J., Reynolds G. T. Temporal sequence and spatial distribution of early events of fertilization in single sea urchin eggs. J Cell Biol. 1984 Nov;99(5):1647–1654. doi: 10.1083/jcb.99.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel D., Weaver A. M., Mazia D. Methods for revoval of the vitelline membrane of sea urchin eggs. I. Use of dithiothreitol (Cleland Reagent). Exp Cell Res. 1970 Jul;61(1):64–68. doi: 10.1016/0014-4827(70)90257-0. [DOI] [PubMed] [Google Scholar]
- Galione A., McDougall A., Busa W. B., Willmott N., Gillot I., Whitaker M. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science. 1993 Jul 16;261(5119):348–352. doi: 10.1126/science.8392748. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Hamaguchi M. S. Simultaneous investigation of intracellular Ca2+ increase and morphological events upon fertilization in the sand dollar egg. Cell Struct Funct. 1990 Jun;15(3):159–162. doi: 10.1247/csf.15.159. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Itoh H., Ishiwata S., Hirano K., Nishizaka T., Hayakawa T. Dual-view microscopy with a single camera: real-time imaging of molecular orientations and calcium. J Cell Biol. 1991 Oct;115(1):67–73. doi: 10.1083/jcb.115.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. Dynamic structure of biological and model membranes: analysis by optical anisotropy decay measurement. Adv Biophys. 1984;17:147–203. doi: 10.1016/0065-227x(84)90027-3. [DOI] [PubMed] [Google Scholar]
- McCulloh D. H., Chambers E. L. Fusion of membranes during fertilization. Increases of the sea urchin egg's membrane capacitance and membrane conductance at the site of contact with the sperm. J Gen Physiol. 1992 Feb;99(2):137–175. doi: 10.1085/jgp.99.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri T., Hamaguchi Y. Propagation of transient Ca2+ increase in sea urchin eggs upon fertilization and its regulation by microinjecting EGTA solution. Cell Struct Funct. 1991 Apr;16(2):157–165. doi: 10.1247/csf.16.157. [DOI] [PubMed] [Google Scholar]
- Paul M., Epel D. Fertilization-associated light-scattering changes in eggs of the sea urchin Strongylocentrotus purpuratus. Exp Cell Res. 1971 Apr;65(2):281–288. doi: 10.1016/0014-4827(71)90003-6. [DOI] [PubMed] [Google Scholar]
- Payan P., Girard J. P., Ciapa B. Mechanisms regulating intracellular pH in sea urchin eggs. Dev Biol. 1983 Nov;100(1):29–38. doi: 10.1016/0012-1606(83)90197-5. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. Microvilli on sea urchin eggs: a second burst of elongation. Dev Biol. 1978 Jun;64(2):342–346. doi: 10.1016/0012-1606(78)90085-4. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. Surface area change at fertilization: resorption of the mosaic membrane. Dev Biol. 1979 Jun;70(2):306–326. doi: 10.1016/0012-1606(79)90030-7. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature. 1978 Mar 16;272(5650):253–254. doi: 10.1038/272253a0. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Lundin L., Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker S. A., Centonze V. E., Paddock S. W., Schatten G. Confocal microscopy of fertilization-induced calcium dynamics in sea urchin eggs. Dev Biol. 1992 Feb;149(2):370–380. doi: 10.1016/0012-1606(92)90292-o. [DOI] [PubMed] [Google Scholar]
- Swann K., Whitaker M. Stimulation of the Na/H exchanger of sea urchin eggs by phorbol ester. Nature. 1985 Mar 21;314(6008):274–277. doi: 10.1038/314274a0. [DOI] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. The relation between the increase in reduced nicotinamide nucleotides and the initiation of DNA synthesis in sea urchin eggs. Cell. 1981 Jul;25(1):95–103. doi: 10.1016/0092-8674(81)90234-8. [DOI] [PubMed] [Google Scholar]