Abstract
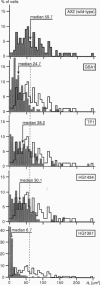
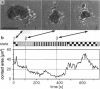
Reflection interference contrast microscopy combined with digital image processing was applied to study the motion of Dictyostelium discoideum cells in their pre-aggregative state on substrata of different adhesiveness (glass, albumin-covered glass, and freshly cleaved mica). The temporal variations of the size and shape of the cell/substratum contact area and the time course of advancement of pseudopods protruding in contact with the substratum were analyzed. The major goal was to study differences between the locomotion of wild-type cells and strains of triple mutants deficient in two F-actin cross-linking proteins (alpha-actinin and the 120-kDa gelation factor) and one F-actin fragmenting protein (severin). The size of contact area, AC, of both wild-type and mutant cells fluctuates between minimum and maximum values on the order of minutes, pointing toward an intrinsic switching mechanism associated with the mechanochemical control system. The fluctuation amplitudes are much larger on freshly cleaved mica than on glass. Wild-type and mutant cells exhibit remarkable differences on mica but not on glass. These differences comprise the population median of AC and alterations in pseudopod protrusion. AC is smaller by a factor of two or more for all mutants. Pseudopods protrude slower and shorter in the mutants. It is concluded that cell shape and pseudopods are destabilized by defects in the actin-skeleton, which can be overcompensated by strongly adhesive substrata. Several features of amoeboid cell locomotion on substrata can be understood on the basis of the minimum bending energy concept of soft adhering shells and by assuming that adhesion induces local alterations of the composite membrane consisting of the protein/lipid bilayer on the cell surface and the underlying actin-cortex.
Full text
PDF

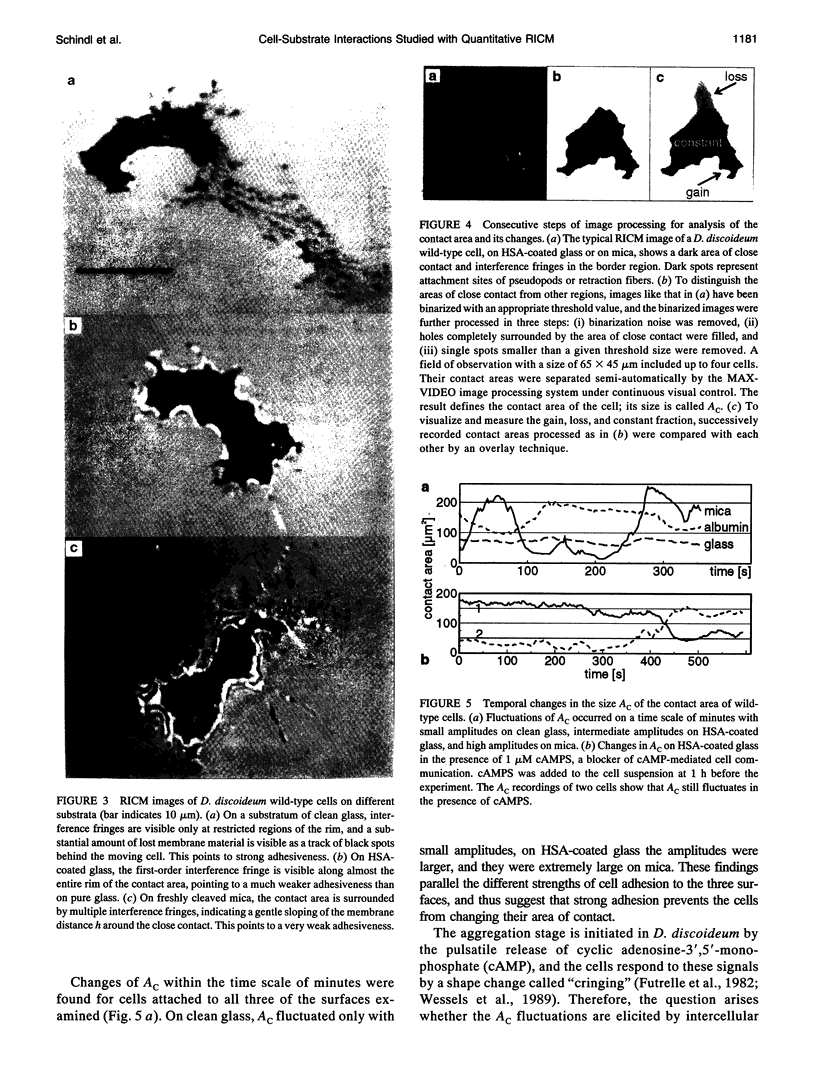
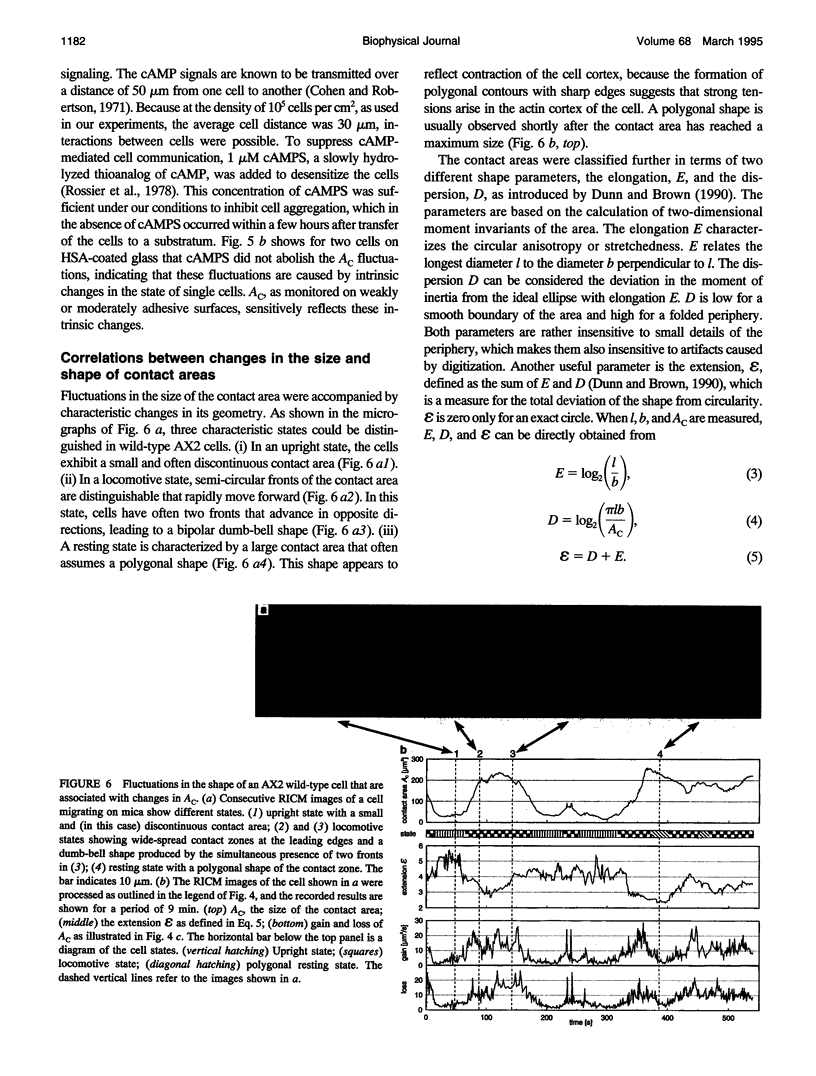
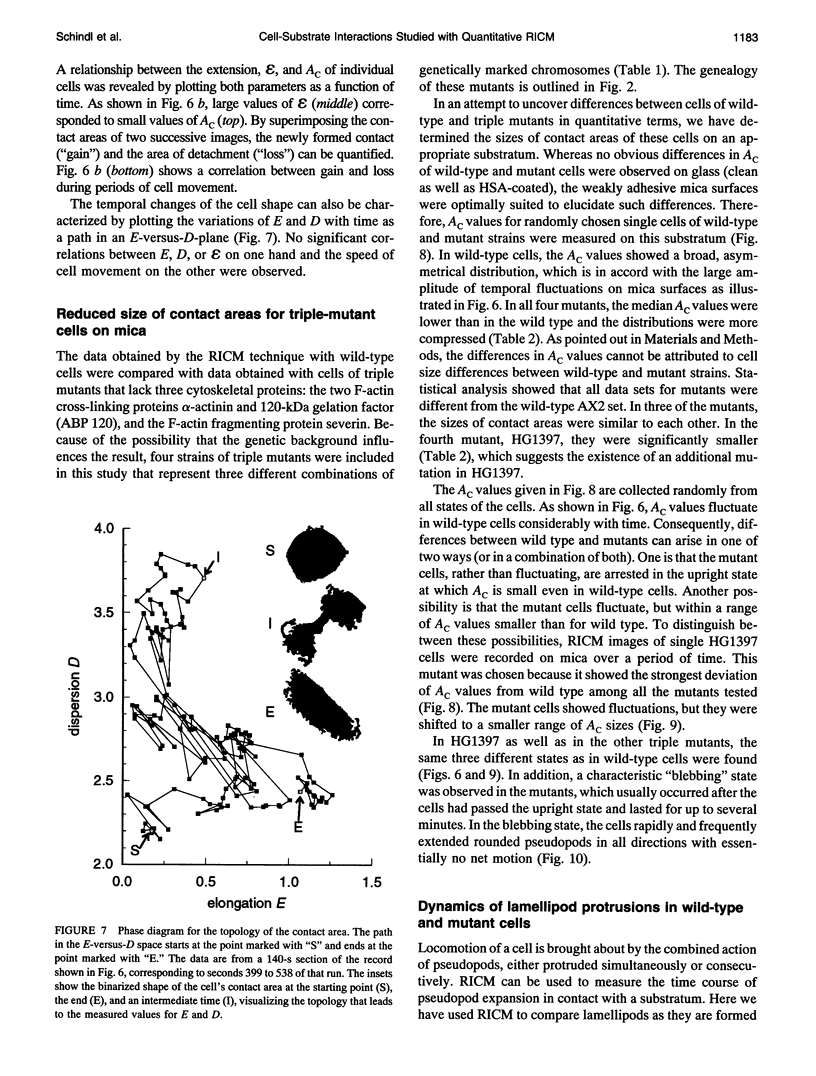
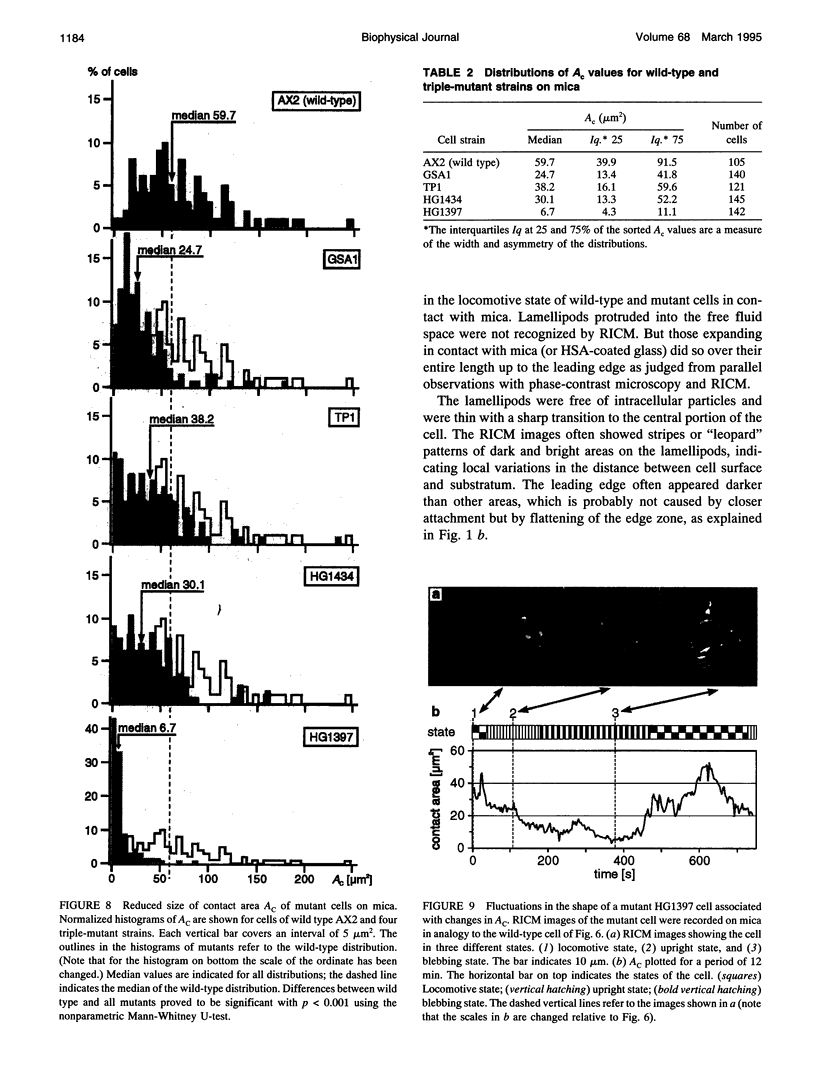





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André E., Brink M., Gerisch G., Isenberg G., Noegel A., Schleicher M., Segall J. E., Wallraff E. A Dictyostelium mutant deficient in severin, an F-actin fragmenting protein, shows normal motility and chemotaxis. J Cell Biol. 1989 Mar;108(3):985–995. doi: 10.1083/jcb.108.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink M., Gerisch G., Isenberg G., Noegel A. A., Segall J. E., Wallraff E., Schleicher M. A Dictyostelium mutant lacking an F-actin cross-linking protein, the 120-kD gelation factor. J Cell Biol. 1990 Oct;111(4):1477–1489. doi: 10.1083/jcb.111.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. S., Yamamoto K., Spudich J. A. A 40,000-dalton protein from Dictyostelium discoideum affects assembly properties of actin in a Ca2+-dependent manner. J Cell Biol. 1982 Apr;93(1):205–210. doi: 10.1083/jcb.93.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS A. S. THE MECHANISM OF ADHESION OF CELLS TO GLASS. A STUDY BY INTERFERENCE REFLECTION MICROSCOPY. J Cell Biol. 1964 Feb;20:199–215. doi: 10.1083/jcb.20.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. H., Robertson A. Chemotaxis and the early stages of aggregation in cellular slime molds. J Theor Biol. 1971 Apr;31(1):119–130. doi: 10.1016/0022-5193(71)90125-1. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Condeelis J., Vahey M. A calcium- and pH-regulated protein from Dictyostelium discoideum that cross-links actin filaments. J Cell Biol. 1982 Aug;94(2):466–471. doi: 10.1083/jcb.94.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J., Vahey M., Carboni J. M., DeMey J., Ogihara S. Properties of the 120,000- and 95,000-dalton actin-binding proteins from Dictyostelium discoideum and their possible functions in assembling the cytoplasmic matrix. J Cell Biol. 1984 Jul;99(1 Pt 2):119s–126s. doi: 10.1083/jcb.99.1.119s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Bryan J., Schwab B., 3rd, Frieden C., Loftus D. J., Elson E. L. Microinjection of gelsolin into living cells. J Cell Biol. 1987 Mar;104(3):491–501. doi: 10.1083/jcb.104.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D., Condeelis J., Wessels D., Soll D., Kern H., Knecht D. A. Targeted disruption of the ABP-120 gene leads to cells with altered motility. J Cell Biol. 1992 Feb;116(4):943–955. doi: 10.1083/jcb.116.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. C., Gorlin J. B., Kwiatkowski D. J., Hartwig J. H., Janmey P. A., Byers H. R., Stossel T. P. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992 Jan 17;255(5042):325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., Stossel T. P., Kwiatkowski D. J. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991 Mar 8;251(4998):1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Lee R. J., Spudich J. A. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993 Oct 22;75(2):363–371. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- Evans E. New physical concepts for cell amoeboid motion. Biophys J. 1993 Apr;64(4):1306–1322. doi: 10.1016/S0006-3495(93)81497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. R., Merkl R., Gerisch G. Quantitative analysis of cell motility and chemotaxis in Dictyostelium discoideum by using an image processing system and a novel chemotaxis chamber providing stationary chemical gradients. J Cell Biol. 1989 Mar;108(3):973–984. doi: 10.1083/jcb.108.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futrelle R. P., Traut J., McKee W. G. Cell behavior in Dictyostelium discoideum: preaggregation response to localized cyclic AMP pulses. J Cell Biol. 1982 Mar;92(3):807–821. doi: 10.1083/jcb.92.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Albrecht R., De Hostos E., Wallraff E., Heizer C., Kreitmeier M., Müller-Taubenberger A. Actin-associated proteins in motility and chemotaxis of Dictyostelium cells. Symp Soc Exp Biol. 1993;47:297–315. [PubMed] [Google Scholar]
- Gerisch G., Keller H. U. Chemotactic reorientation of granulocytes stimulated with micropipettes containing fMet-Leu-Phe. J Cell Sci. 1981 Dec;52:1–10. doi: 10.1242/jcs.52.1.1. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Wick U. Intracellular oscillations and release of cyclic AMP from Dictyostelium cells. Biochem Biophys Res Commun. 1975 Jul 8;65(1):364–370. doi: 10.1016/s0006-291x(75)80102-1. [DOI] [PubMed] [Google Scholar]
- Gingell D., Todd I. Interference reflection microscopy. A quantitative theory for image interpretation and its application to cell-substratum separation measurement. Biophys J. 1979 Jun;26(3):507–526. doi: 10.1016/S0006-3495(79)85268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin J. B., Yamin R., Egan S., Stewart M., Stossel T. P., Kwiatkowski D. J., Hartwig J. H. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990 Sep;111(3):1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. K., Wild P., Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980 Apr 11;208(4440):177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Goldmann W. H. Actin-membrane coupling: a role for talin. J Muscle Res Cell Motil. 1992 Dec;13(6):587–589. doi: 10.1007/BF01738248. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Hvidt S., Lamb J., Stossel T. P. Resemblance of actin-binding protein/actin gels to covalently crosslinked networks. Nature. 1990 May 3;345(6270):89–92. doi: 10.1038/345089a0. [DOI] [PubMed] [Google Scholar]
- Janmey P. A. Mechanical properties of cytoskeletal polymers. Curr Opin Cell Biol. 1991 Feb;3(1):4–11. doi: 10.1016/0955-0674(91)90159-v. [DOI] [PubMed] [Google Scholar]
- Jung G., Hammer J. A., 3rd Generation and characterization of Dictyostelium cells deficient in a myosin I heavy chain isoform. J Cell Biol. 1990 Jun;110(6):1955–1964. doi: 10.1083/jcb.110.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth A., von Arnim V., Biegelmann E., Humbel B., Schweiger A., Gerisch G. Strong increase in the tyrosine phosphorylation of actin upon inhibition of oxidative phosphorylation: correlation with reversible rearrangements in the actin skeleton of Dictyostelium cells. J Cell Sci. 1994 Jan;107(Pt 1):117–125. doi: 10.1242/jcs.107.1.117. [DOI] [PubMed] [Google Scholar]
- Kayman S. C., Clarke M. Relationship between axenic growth of Dictyostelium discoideum strains and their track morphology on substrates coated with gold particles. J Cell Biol. 1983 Oct;97(4):1001–1010. doi: 10.1083/jcb.97.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killich T., Plath P. J., Wei X., Bultmann H., Rensing L., Vicker M. G. The locomotion, shape and pseudopodial dynamics of unstimulated Dictyostelium cells are not random. J Cell Sci. 1993 Dec;106(Pt 4):1005–1013. doi: 10.1242/jcs.106.4.1005. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Kolodney M. S., Elson E. L. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993 Nov 15;268(32):23850–23855. [PubMed] [Google Scholar]
- Lee J., Ishihara A., Jacobson K. How do cells move along surfaces? Trends Cell Biol. 1993 Nov;3(11):366–370. doi: 10.1016/0962-8924(93)90084-e. [DOI] [PubMed] [Google Scholar]
- Lee J., Ishihara A., Theriot J. A., Jacobson K. Principles of locomotion for simple-shaped cells. Nature. 1993 Mar 11;362(6416):167–171. doi: 10.1038/362167a0. [DOI] [PubMed] [Google Scholar]
- Manstein D. J., Titus M. A., De Lozanne A., Spudich J. A. Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 1989 Mar;8(3):923–932. doi: 10.1002/j.1460-2075.1989.tb03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz B. A., Ward T. E., Welker D. L., Williams K. L. Identification of an endogenous plasmid in Dictyostelium discoideum. EMBO J. 1983;2(4):515–519. doi: 10.1002/j.1460-2075.1983.tb01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A. A., Rapp S., Lottspeich F., Schleicher M., Stewart M. The Dictyostelium gelation factor shares a putative actin binding site with alpha-actinins and dystrophin and also has a rod domain containing six 100-residue motifs that appear to have a cross-beta conformation. J Cell Biol. 1989 Aug;109(2):607–618. doi: 10.1083/jcb.109.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster G. F., Perelson A. S. The physics of cell motility. J Cell Sci Suppl. 1987;8:35–54. doi: 10.1242/jcs.1987.supplement_8.3. [DOI] [PubMed] [Google Scholar]
- Pasternak C., Spudich J. A., Elson E. L. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989 Oct 12;341(6242):549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Rossier C., Gerisch G., Malchow D. Action of a slowly hydrolysable cyclic AMP analogue on developing cells of Dictyostelium discoideum. J Cell Sci. 1979 Feb;35:321–338. doi: 10.1242/jcs.35.1.321. [DOI] [PubMed] [Google Scholar]
- Ruddies R., Goldmann W. H., Isenberg G., Sackmann E. The viscoelasticity of entangled actin networks: the influence of defects and modulation by talin and vinculin. Eur Biophys J. 1993;22(5):309–321. doi: 10.1007/BF00213554. [DOI] [PubMed] [Google Scholar]
- Schleicher M., Noegel A., Schwarz T., Wallraff E., Brink M., Faix J., Gerisch G., Isenberg G. A Dictyostelium mutant with severe defects in alpha-actinin: its characterization using cDNA probes and monoclonal antibodies. J Cell Sci. 1988 May;90(Pt 1):59–71. doi: 10.1242/jcs.90.1.59. [DOI] [PubMed] [Google Scholar]
- Seifert U, Lipowsky R. Adhesion of vesicles. Phys Rev A. 1990 Oct 15;42(8):4768–4771. doi: 10.1103/physreva.42.4768. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S. Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol. 1979;56:57–144. doi: 10.1016/s0074-7696(08)61821-5. [DOI] [PubMed] [Google Scholar]
- Titus M. A., Wessels D., Spudich J. A., Soll D. The unconventional myosin encoded by the myoA gene plays a role in Dictyostelium motility. Mol Biol Cell. 1993 Feb;4(2):233–246. doi: 10.1091/mbc.4.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik K. J., Devreotes P. N. Adenosine 3',5'-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution--fluorography. Science. 1981 Apr 24;212(4493):443–446. doi: 10.1126/science.6259734. [DOI] [PubMed] [Google Scholar]
- Verschueren H. Interference reflection microscopy in cell biology: methodology and applications. J Cell Sci. 1985 Apr;75:279–301. doi: 10.1242/jcs.75.1.279. [DOI] [PubMed] [Google Scholar]
- Vince S., Gingell D. Cationic modulation of the interaction of Dictyostelium discoideum amoebae with glass. Evidence from quantitative interference reflection microscopy. Exp Cell Res. 1980 Apr;126(2):462–465. doi: 10.1016/0014-4827(80)90288-8. [DOI] [PubMed] [Google Scholar]
- Wallraff E., Gerisch G. Screening for Dictyostelium mutants defective in cytoskeletal proteins by colony immunoblotting. Methods Enzymol. 1991;196:334–348. doi: 10.1016/0076-6879(91)96030-u. [DOI] [PubMed] [Google Scholar]
- Wallraff E., Schleicher M., Modersitzki M., Rieger D., Isenberg G., Gerisch G. Selection of Dictyostelium mutants defective in cytoskeletal proteins: use of an antibody that binds to the ends of alpha-actinin rods. EMBO J. 1986 Jan;5(1):61–67. doi: 10.1002/j.1460-2075.1986.tb04178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D., Schroeder N. A., Voss E., Hall A. L., Condeelis J., Soll D. R. cAMP-mediated inhibition of intracellular particle movement and actin reorganization in Dictyostelium. J Cell Biol. 1989 Dec;109(6 Pt 1):2841–2851. doi: 10.1083/jcb.109.6.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D., Vawter-Hugart H., Murray J., Soll D. R. Three-dimensional dynamics of pseudopod formation and the regulation of turning during the motility cycle of Dictyostelium. Cell Motil Cytoskeleton. 1994;27(1):1–12. doi: 10.1002/cm.970270102. [DOI] [PubMed] [Google Scholar]
- Williams K. L. Characterization of Dominant Resistance to Cobalt Chloride in DICTYOSTELIUM DISCOIDEUM and Its Use in Parasexual Genetic Analysis. Genetics. 1978 Sep;90(1):37–47. doi: 10.1093/genetics/90.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. L., Newell P. C. A genetic study of aggregation in the cellular slime mould Dictyostelium discoideum using complementation analysis. Genetics. 1976 Feb;82(2):287–307. doi: 10.1093/genetics/82.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Nellen W., Noegel A. Homologous recombination in the Dictyostelium alpha-actinin gene leads to an altered mRNA and lack of the protein. EMBO J. 1987 Dec 20;6(13):4143–4148. doi: 10.1002/j.1460-2075.1987.tb02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Schleicher M., Noegel A. A. Redundancy in the microfilament system: abnormal development of Dictyostelium cells lacking two F-actin cross-linking proteins. Cell. 1992 Jan 10;68(1):53–62. doi: 10.1016/0092-8674(92)90205-q. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Pardee J. D., Reidler J., Stryer L., Spudich J. A. Mechanism of interaction of Dictyostelium severin with actin filaments. J Cell Biol. 1982 Dec;95(3):711–719. doi: 10.1083/jcb.95.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilker A, Ziegler M, Sackmann E. Spectral analysis of erythrocyte flickering in the 0.3-4- microm-1 regime by microinterferometry combined with fast image processing. Phys Rev A. 1992 Dec 15;46(12):7998–8001. doi: 10.1103/physreva.46.7998. [DOI] [PubMed] [Google Scholar]
- de Hostos E. L., Rehfuess C., Bradtke B., Waddell D. R., Albrecht R., Murphy J., Gerisch G. Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. J Cell Biol. 1993 Jan;120(1):163–173. doi: 10.1083/jcb.120.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]