Abstract
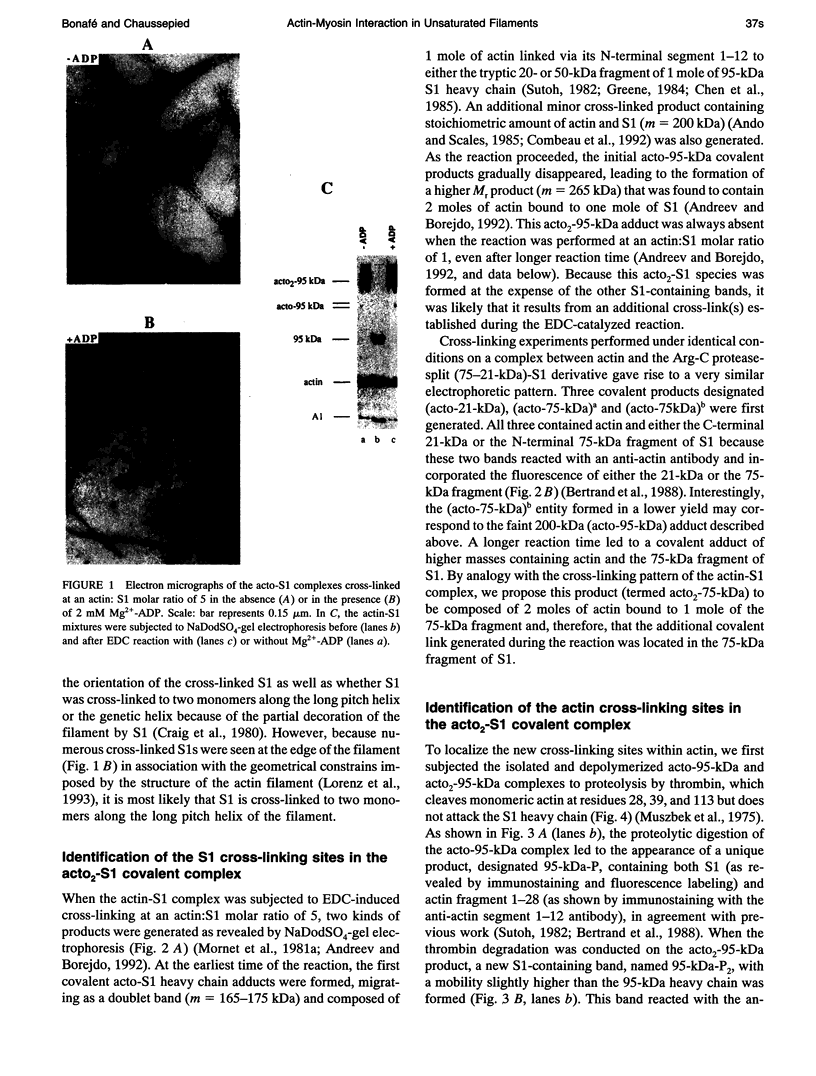
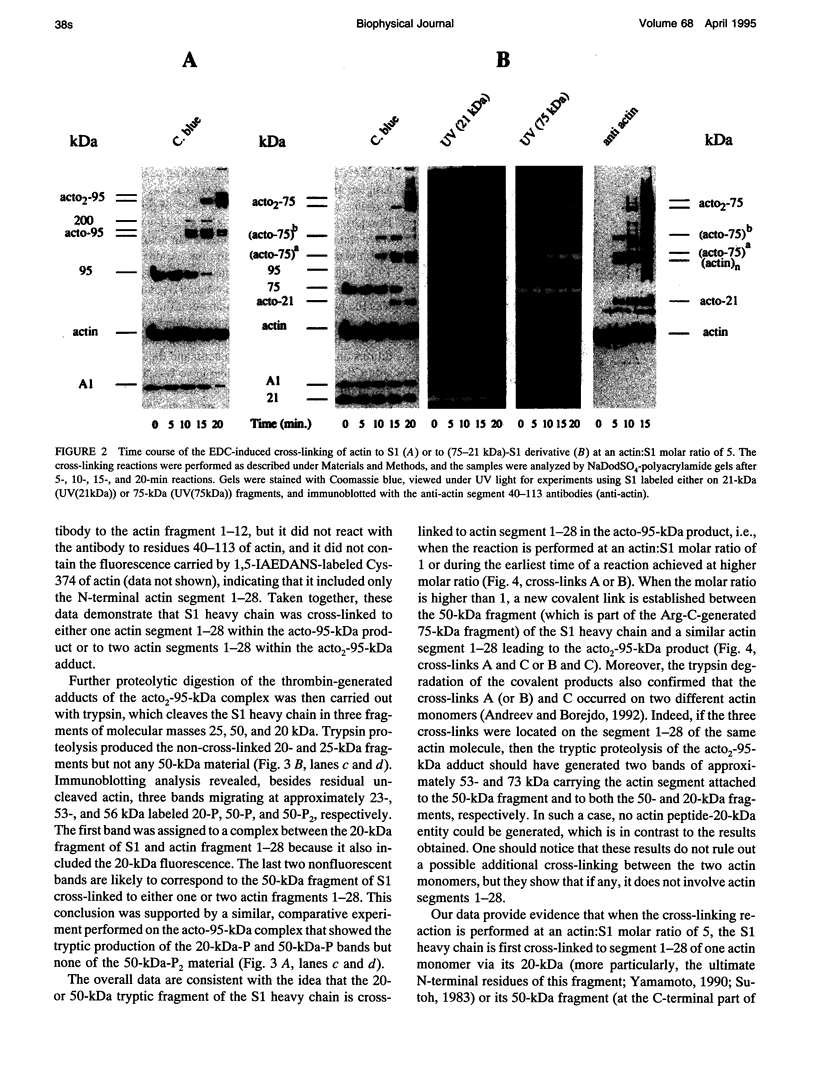
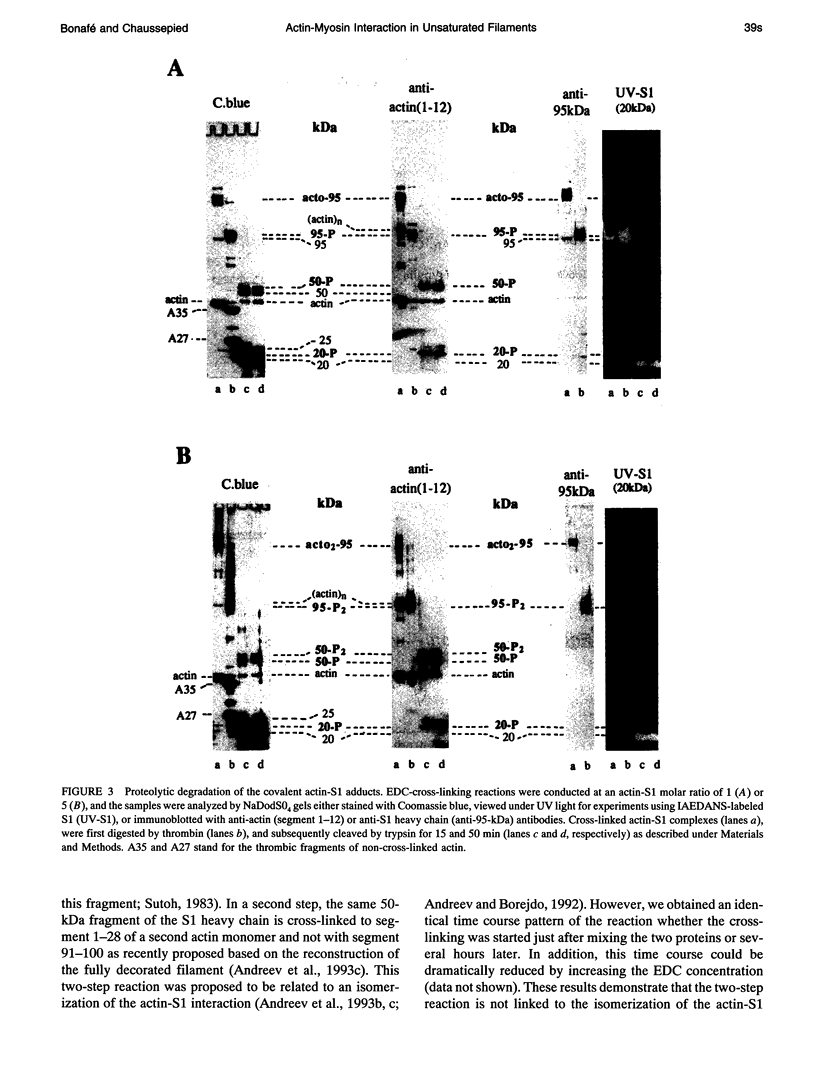
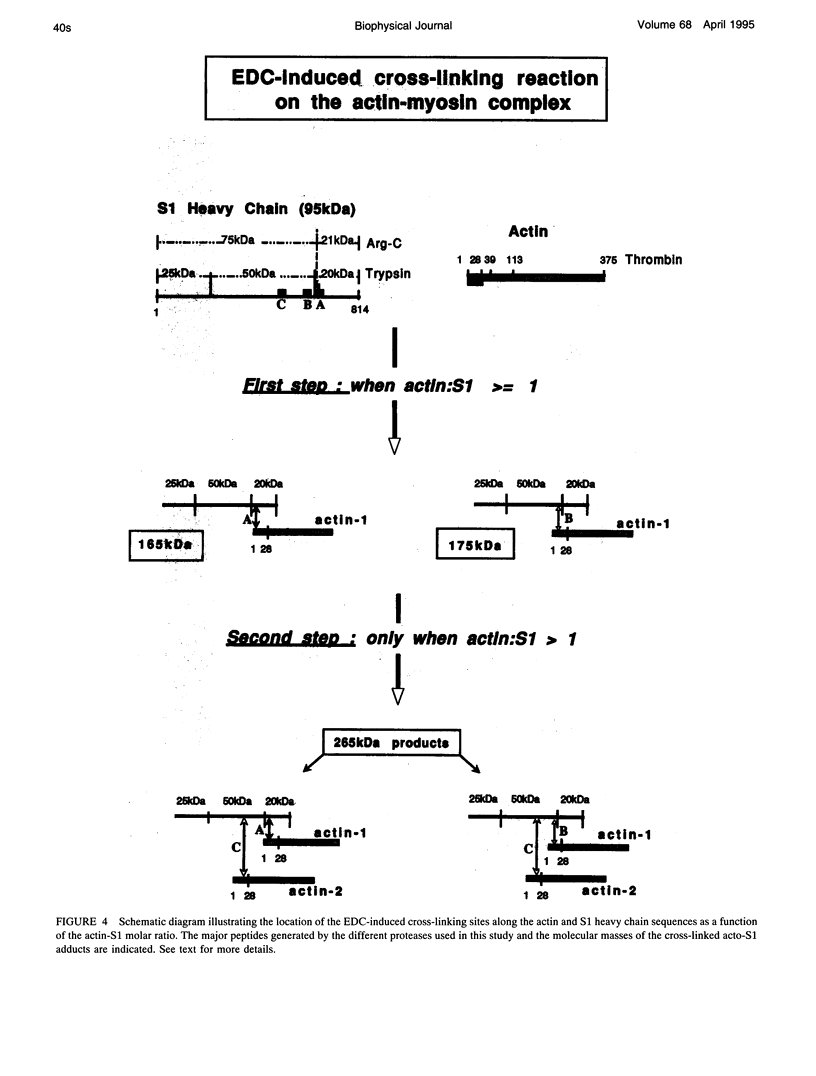
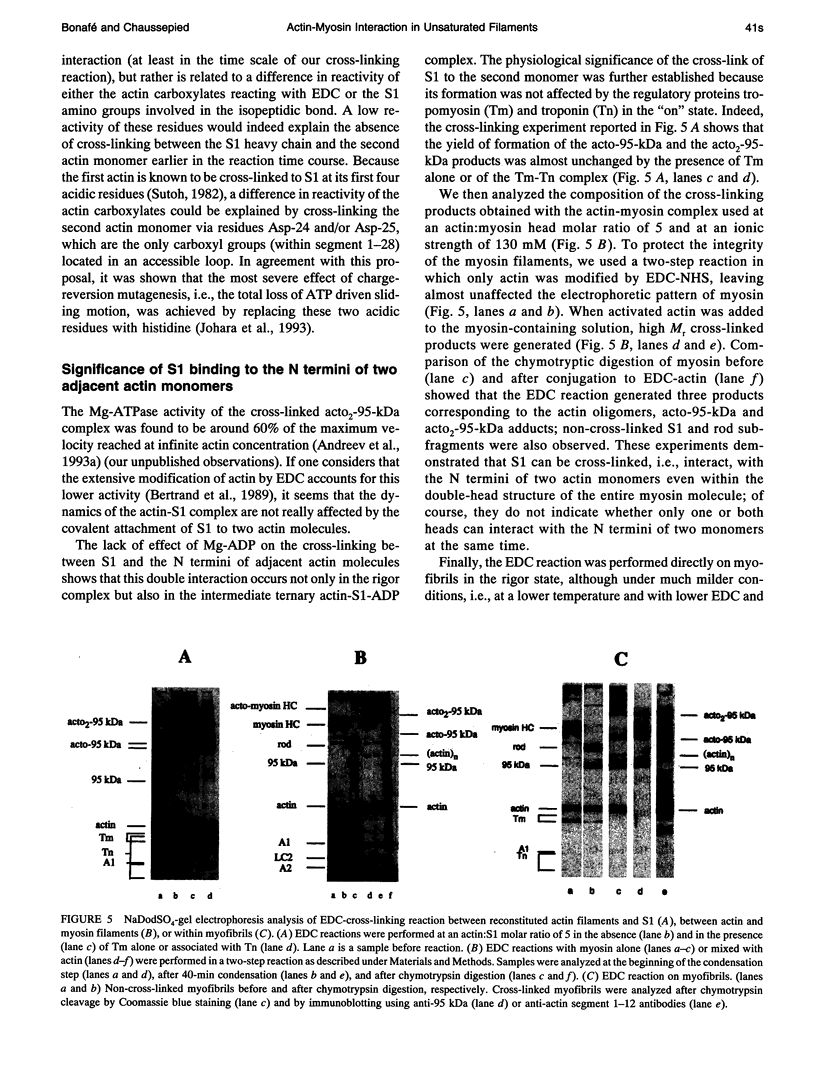
Myosin subfragment-1 (S1) can be cross-linked to two actin monomers by 1-ethyl-3-[3-(dimethylamino)-propyl]-carbodiimide only when F-actin is in excess over S1. Electron micrographs of the covalent actin2-S1 complex showed that S1 was cross-linked to two adjacent monomers of the same actin filament. Cross-linking experiments with pre-proteolyzed S1 derivatives in combination with a proteolytic dissection of the intact covalent actin2-S1 adduct (m = 265 kDa), revealed that two N-terminal segments of actin (residues 1-28) were covalently attached to a single S1 molecule. One was cross-linked to either the 20-kDa or the 50-kDa heavy chain fragments of S1, and the other only to the 50-kDa region. The doubly cross-linked product was formed under physiological ionic strength with S1 or with reconstituted myosin filaments, regardless of the presence of ADP or the regulatory proteins, tropomyosin and troponin. Finally, we found that this cross-linking could also take place within myofibrils in the rigor state. These results demonstrate that under nonsaturating conditions, the actin-S1 interface encompasses a much larger region than that recently proposed for the nonphysiological, fully saturated actin filaments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. Bundling of myosin subfragment-1-decorated actin filaments. J Mol Biol. 1987 May 20;195(2):351–358. doi: 10.1016/0022-2836(87)90656-5. [DOI] [PubMed] [Google Scholar]
- Ando T., Scales D. Skeletal muscle myosin subfragment-1 induces bundle formation by actin filaments. J Biol Chem. 1985 Feb 25;260(4):2321–2327. [PubMed] [Google Scholar]
- Andreev O. A., Andreeva A. L., Borejdo J. Polarization of fluorescently labeled myosin subfragment-1 fully or partially decorating muscle fibers and myofibrils. Biophys J. 1993 Sep;65(3):1027–1038. doi: 10.1016/S0006-3495(93)81161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev O. A., Andreeva A. L., Markin V. S., Borejdo J. Two different rigor complexes of myosin subfragment 1 and actin. Biochemistry. 1993 Nov 16;32(45):12046–12053. doi: 10.1021/bi00096a015. [DOI] [PubMed] [Google Scholar]
- Andreev O. A., Borejdo J. Two different acto-S1 complexes. J Muscle Res Cell Motil. 1992 Oct;13(5):523–533. doi: 10.1007/BF01737995. [DOI] [PubMed] [Google Scholar]
- Andreeva A. L., Andreev O. A., Borejdo J. Structure of the 265-kilodalton complex formed upon EDC cross-linking of subfragment 1 to F-actin. Biochemistry. 1993 Dec 21;32(50):13956–13960. doi: 10.1021/bi00213a027. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Chaussepied P., Audemard E., Kassab R. Functional characterization of skeletal F-actin labeled on the NH2-terminal segment of residues 1-28. Eur J Biochem. 1989 May 15;181(3):747–754. doi: 10.1111/j.1432-1033.1989.tb14787.x. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Chaussepied P., Kassab R., Boyer M., Roustan C., Benyamin Y. Cross-linking of the skeletal myosin subfragment 1 heavy chain to the N-terminal actin segment of residues 40-113. Biochemistry. 1988 Jul 26;27(15):5728–5736. doi: 10.1021/bi00415a050. [DOI] [PubMed] [Google Scholar]
- Bonafé N., Chaussepied P., Capony J. P., Derancourt J., Kassab R. Photochemical cross-linking of the skeletal myosin head heavy chain to actin subdomain-1 at Arg95 and Arg28. Eur J Biochem. 1993 May 1;213(3):1243–1254. doi: 10.1111/j.1432-1033.1993.tb17875.x. [DOI] [PubMed] [Google Scholar]
- Bonafé N., Mathieu M., Kassab R., Chaussepied P. Tropomyosin inhibits the glutaraldehyde-induced cross-link between the central 48-kDa fragment of myosin head and segment 48-67 in actin subdomain 2. Biochemistry. 1994 Mar 8;33(9):2594–2603. doi: 10.1021/bi00175a031. [DOI] [PubMed] [Google Scholar]
- Botts J., Thomason J. F., Morales M. F. On the origin and transmission of force in actomyosin subfragment 1. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2204–2208. doi: 10.1073/pnas.86.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Kumar S., Titani K., Hauschka S. D. Peptide antibody specific for the amino terminus of skeletal muscle alpha-actin. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1506–1510. doi: 10.1073/pnas.80.6.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Applegate D., Reisler E. Cross-linking of actin to myosin subfragment 1: course of reaction and stoichiometry of products. Biochemistry. 1985 Jan 1;24(1):137–144. doi: 10.1021/bi00322a019. [DOI] [PubMed] [Google Scholar]
- Combeau C., Didry D., Carlier M. F. Interaction between G-actin and myosin subfragment-1 probed by covalent cross-linking. J Biol Chem. 1992 Jul 15;267(20):14038–14046. [PubMed] [Google Scholar]
- Craig R., Megerman J. Assembly of smooth muscle myosin into side-polar filaments. J Cell Biol. 1977 Dec;75(3):990–996. doi: 10.1083/jcb.75.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R., Szent-Györgyi A. G., Beese L., Flicker P., Vibert P., Cohen C. Electron microscopy of thin filaments decorated with a Ca2+-regulated myosin. J Mol Biol. 1980 Jun 15;140(1):35–55. doi: 10.1016/0022-2836(80)90355-1. [DOI] [PubMed] [Google Scholar]
- Greene L. E. Stoichiometry of actin X S-1 cross-linked complex. J Biol Chem. 1984 Jun 25;259(12):7363–7366. [PubMed] [Google Scholar]
- Herrmann C., Sleep J., Chaussepied P., Travers F., Barman T. A structural and kinetic study on myofibrils prevented from shortening by chemical cross-linking. Biochemistry. 1993 Jul 20;32(28):7255–7263. doi: 10.1021/bi00079a023. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T. Selective fluorescent labeling of the 50-, 26-, and 20-kilodalton heavy chain segments of myosin ATPase. J Biochem. 1987 Jun;101(6):1457–1462. doi: 10.1093/oxfordjournals.jbchem.a122015. [DOI] [PubMed] [Google Scholar]
- Huang Y. P., Kimura M., Tawada K. Covalent crosslinking of myosin subfragment-1 and heavy meromyosin to actin at various molar ratios: different correlations between ATPase activity and crosslinking extent. J Muscle Res Cell Motil. 1990 Aug;11(4):313–322. doi: 10.1007/BF01766669. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Johara M., Toyoshima Y. Y., Ishijima A., Kojima H., Yanagida T., Sutoh K. Charge-reversion mutagenesis of Dictyostelium actin to map the surface recognized by myosin during ATP-driven sliding motion. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2127–2131. doi: 10.1073/pnas.90.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lheureux K., Forné T., Chaussepied P. Interaction and polymerization of the G-actin-myosin head complex: effect of DNase I. Biochemistry. 1993 Sep 28;32(38):10005–10014. doi: 10.1021/bi00089a016. [DOI] [PubMed] [Google Scholar]
- Lorenz M., Popp D., Holmes K. C. Refinement of the F-actin model against X-ray fiber diffraction data by the use of a directed mutation algorithm. J Mol Biol. 1993 Dec 5;234(3):826–836. doi: 10.1006/jmbi.1993.1628. [DOI] [PubMed] [Google Scholar]
- Mornet D., Bertrand R., Pantel P., Audemard E., Kassab R. Structure of the actin-myosin interface. Nature. 1981 Jul 23;292(5821):301–306. doi: 10.1038/292301a0. [DOI] [PubMed] [Google Scholar]
- Muszbek L., Gladner J. A., Laki K. The fragmentation of actin by thrombin. Isolation and characterization of the split products. Arch Biochem Biophys. 1975 Mar;167(1):99–103. doi: 10.1016/0003-9861(75)90445-2. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Schröder R. R., Manstein D. J., Jahn W., Holden H., Rayment I., Holmes K. C., Spudich J. A. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993 Jul 8;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Mapping of actin-binding sites on the heavy chain of myosin subfragment 1. Biochemistry. 1983 Mar 29;22(7):1579–1585. doi: 10.1021/bi00276a009. [DOI] [PubMed] [Google Scholar]
- Tesi C., Travers F., Barman T. Cryoenzymic studies on actomyosin ATPase. Evidence that the degree of saturation of actin with myosin subfragment 1 affects the kinetics of the binding of ATP. Biochemistry. 1990 Feb 20;29(7):1846–1852. doi: 10.1021/bi00459a026. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. Shift of binding site at the interface between actin and myosin. Biochemistry. 1990 Jan 23;29(3):844–848. doi: 10.1021/bi00455a035. [DOI] [PubMed] [Google Scholar]