Abstract
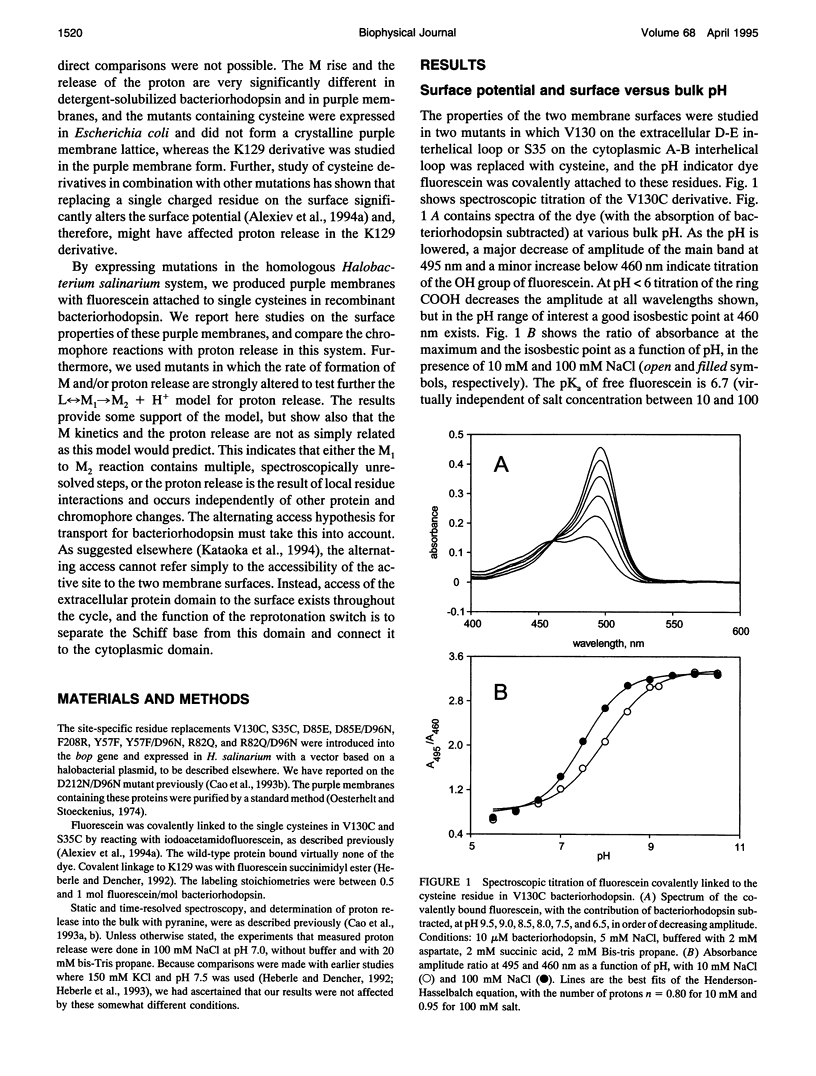
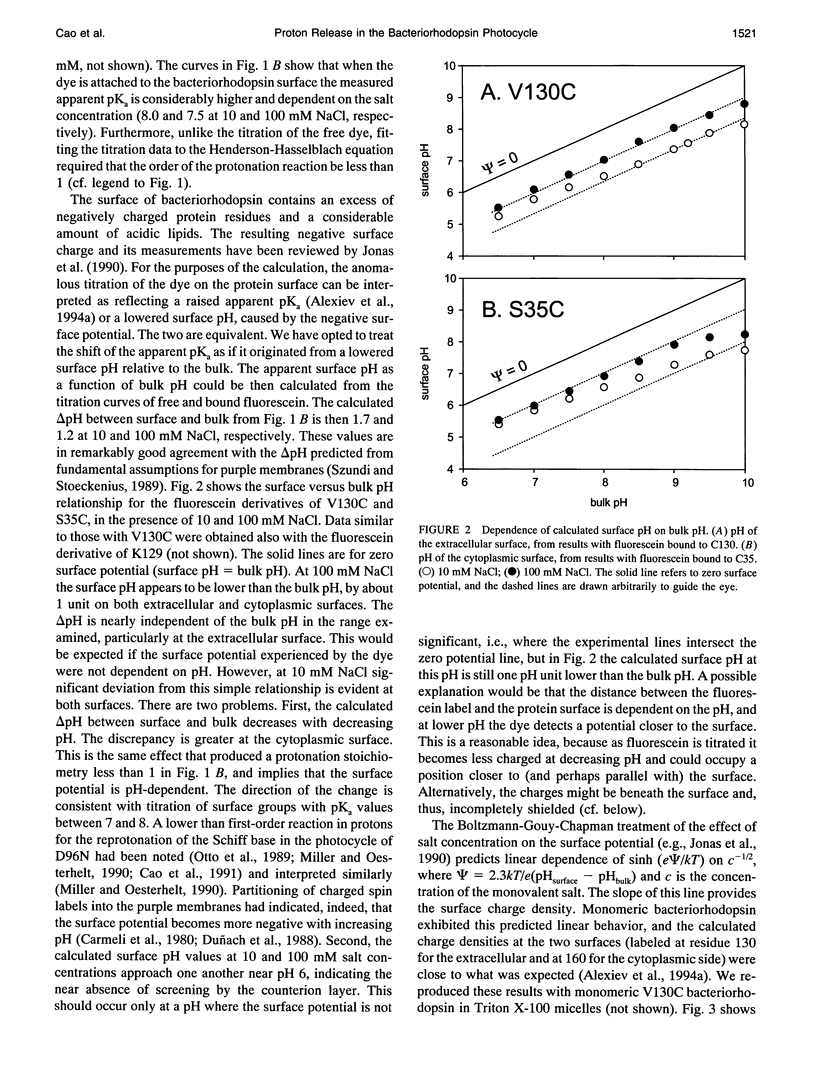
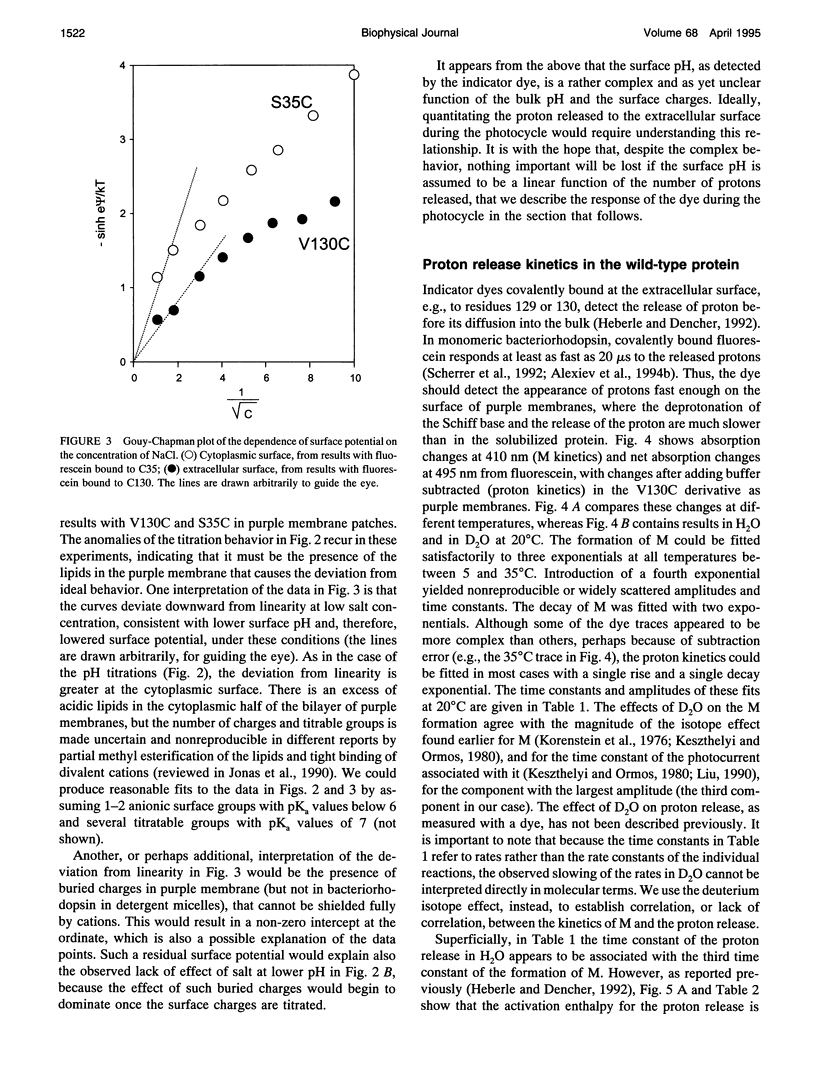
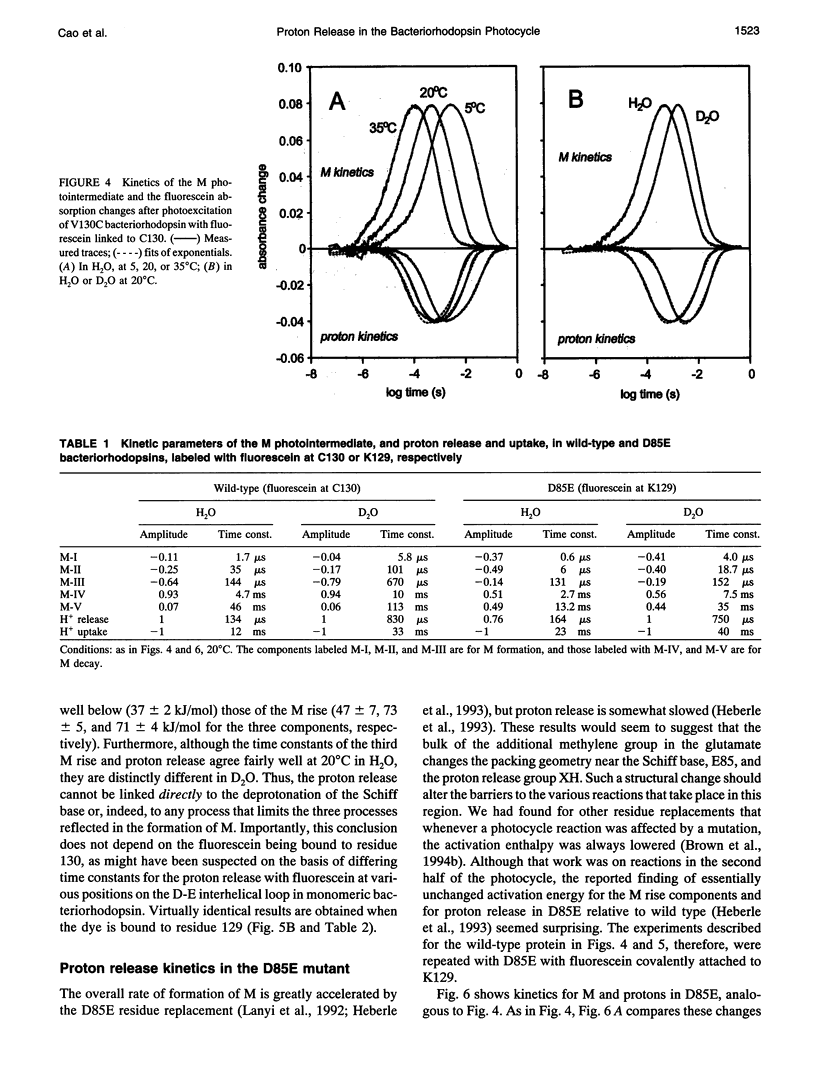
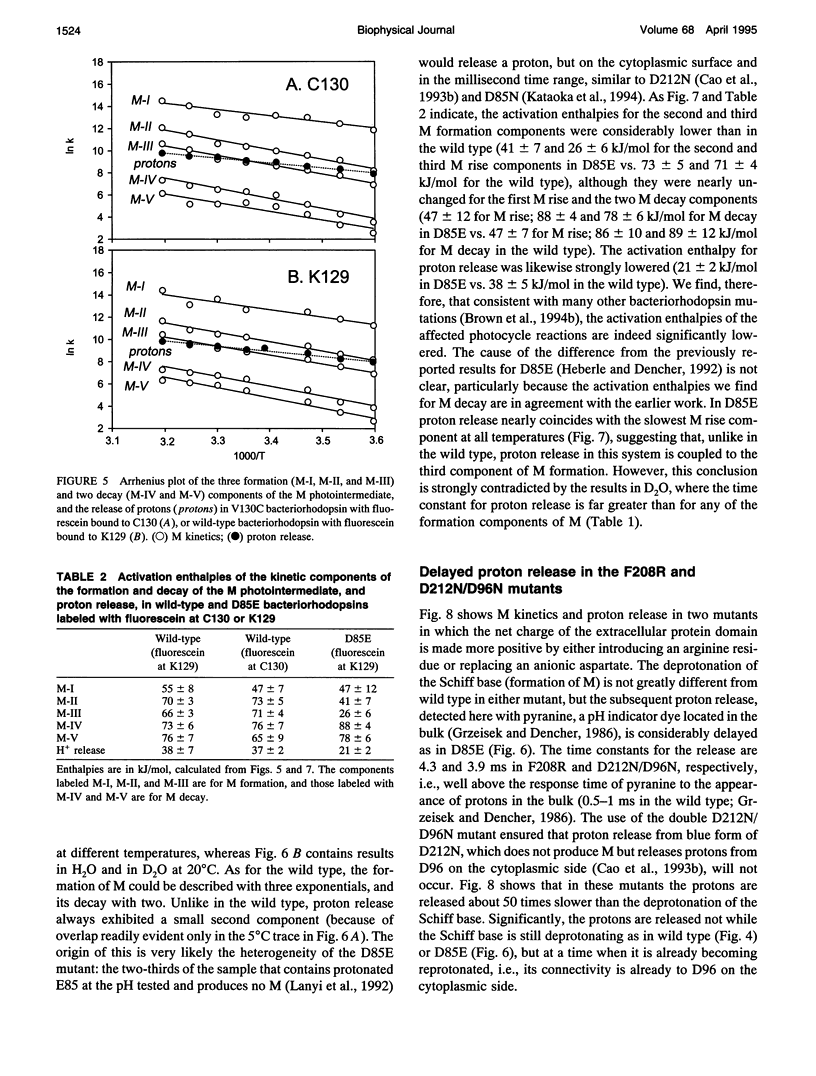
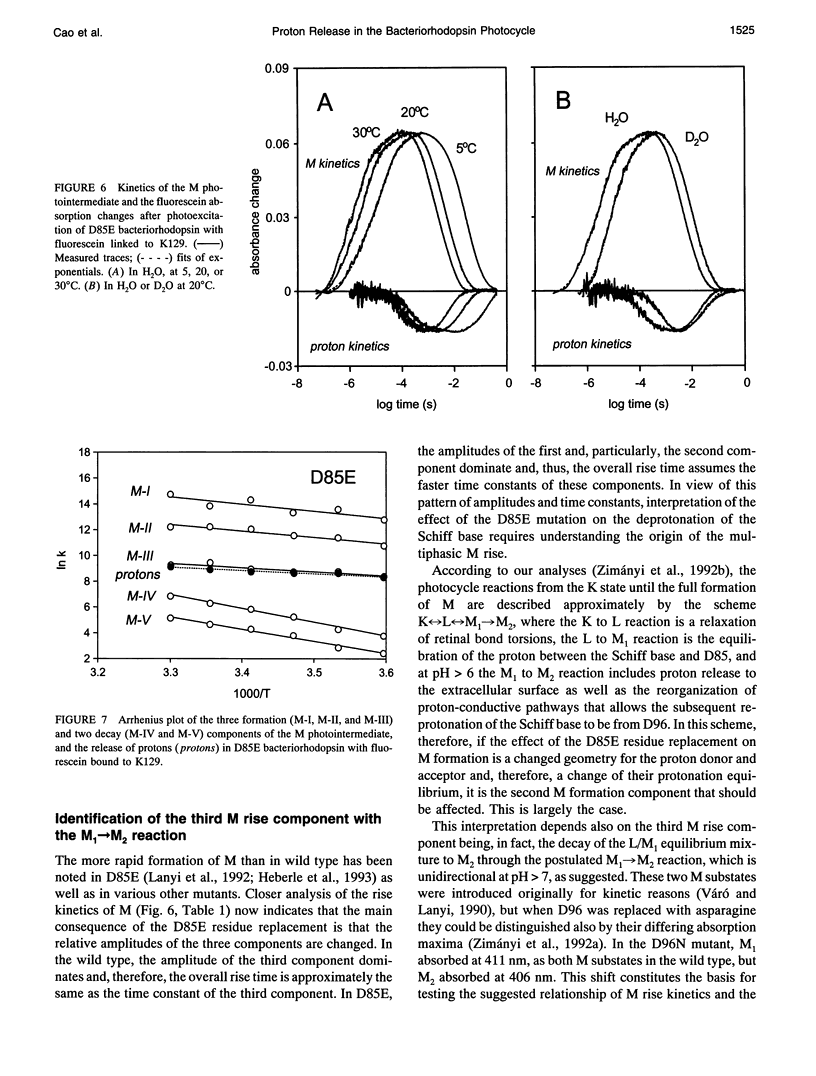
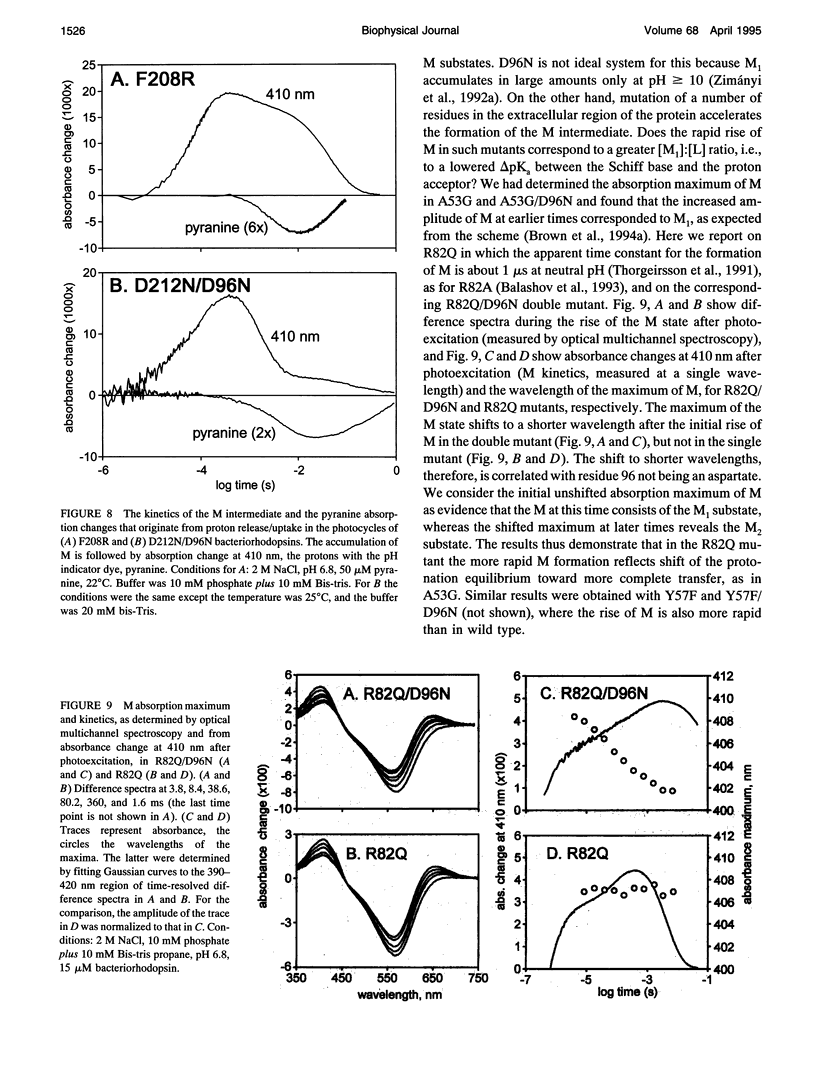
The surface potential of purple membranes and the release of protons during the bacteriorhodopsin photocycle have been studied with the covalently linked pH indicator dye, fluorescein. The titration of acidic lipids appears to cause the surface potential to be pH-dependent and causes other deviations from ideal behavior. If these anomalies are neglected, the appearance of protons can be followed by measuring the absorption change of fluorescein bound to various residues at the extracellular surface. Contrary to widely held assumption, the activation enthalpies of kinetic components, deuterium isotope effects in the time constants, and the consequences of the D85E, F208R, and D212N mutations demonstrate a lack of direct correlation between proton transfer from the buried retinal Schiff base to D85 and proton release at the surface. Depending on conditions and residue replacements, the proton release can occur at any time between the protonation of D85 and the recovery of the initial state. We conclude that once D85 is protonated the proton release at the extracellular protein surface is essentially independent of the chromophore reactions that follow. This finding is consistent with the recently suggested version of the alternating access mechanism of bacteriorhodopsin, in which the change of the accessibility of the Schiff base is to and away from D85 rather than to and away from the extracellular membrane surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexiev U., Marti T., Heyn M. P., Khorana H. G., Scherrer P. Covalently bound pH-indicator dyes at selected extracellular or cytoplasmic sites in bacteriorhodopsin. 2. Rotational orientation of helices D and E and kinetic correlation between M formation and proton release in bacteriorhodopsin micelles. Biochemistry. 1994 Nov 22;33(46):13693–13699. doi: 10.1021/bi00250a020. [DOI] [PubMed] [Google Scholar]
- Alexiev U., Marti T., Heyn M. P., Khorana H. G., Scherrer P. Surface charge of bacteriorhodopsin detected with covalently bound pH indicators at selected extracellular and cytoplasmic sites. Biochemistry. 1994 Jan 11;33(1):298–306. doi: 10.1021/bi00167a039. [DOI] [PubMed] [Google Scholar]
- Balashov S. P., Govindjee R., Kono M., Imasheva E., Lukashev E., Ebrey T. G., Crouch R. K., Menick D. R., Feng Y. Effect of the arginine-82 to alanine mutation in bacteriorhodopsin on dark adaptation, proton release, and the photochemical cycle. Biochemistry. 1993 Oct 5;32(39):10331–10343. doi: 10.1021/bi00090a008. [DOI] [PubMed] [Google Scholar]
- Bousché O., Sonar S., Krebs M. P., Khorana H. G., Rothschild K. J. Time-resolved Fourier transform infrared spectroscopy of the bacteriorhodopsin mutant Tyr-185-->Phe: Asp-96 reprotonates during O formation; Asp-85 and Asp-212 deprotonate during O decay. Photochem Photobiol. 1992 Dec;56(6):1085–1095. doi: 10.1111/j.1751-1097.1992.tb09732.x. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Brown L. S., Bonet L., Needleman R., Lanyi J. K. Estimated acid dissociation constants of the Schiff base, Asp-85, and Arg-82 during the bacteriorhodopsin photocycle. Biophys J. 1993 Jul;65(1):124–130. doi: 10.1016/S0006-3495(93)81064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. S., Gat Y., Sheves M., Yamazaki Y., Maeda A., Needleman R., Lanyi J. K. The retinal Schiff base-counterion complex of bacteriorhodopsin: changed geometry during the photocycle is a cause of proton transfer to aspartate 85. Biochemistry. 1994 Oct 11;33(40):12001–12011. doi: 10.1021/bi00206a001. [DOI] [PubMed] [Google Scholar]
- Brown L. S., Yamazaki Y., Maeda A., Sun L., Needleman R., Lanyi J. K. The proton transfers in the cytoplasmic domain of bacteriorhodopsin are facilitated by a cluster of interacting residues. J Mol Biol. 1994 Jun 10;239(3):401–414. doi: 10.1006/jmbi.1994.1381. [DOI] [PubMed] [Google Scholar]
- Cao Y., Brown L. S., Needleman R., Lanyi J. K. Relationship of proton uptake on the cytoplasmic surface and reisomerization of the retinal in the bacteriorhodopsin photocycle: an attempt to understand the complex kinetics of the pH changes and the N and O intermediates. Biochemistry. 1993 Sep 28;32(38):10239–10248. doi: 10.1021/bi00089a046. [DOI] [PubMed] [Google Scholar]
- Cao Y., Váró G., Chang M., Ni B. F., Needleman R., Lanyi J. K. Water is required for proton transfer from aspartate-96 to the bacteriorhodopsin Schiff base. Biochemistry. 1991 Nov 12;30(45):10972–10979. doi: 10.1021/bi00109a023. [DOI] [PubMed] [Google Scholar]
- Cao Y., Váró G., Klinger A. L., Czajkowsky D. M., Braiman M. S., Needleman R., Lanyi J. K. Proton transfer from Asp-96 to the bacteriorhodopsin Schiff base is caused by a decrease of the pKa of Asp-96 which follows a protein backbone conformational change. Biochemistry. 1993 Mar 2;32(8):1981–1990. doi: 10.1021/bi00059a015. [DOI] [PubMed] [Google Scholar]
- Carmeli C., Quintanilha A. T., Packer L. Surface charge changes in purple membranes and the photoreaction cycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4707–4711. doi: 10.1073/pnas.77.8.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronister E. L., Corcoran T. C., Song L., El-Sayed M. A. On the molecular mechanisms of the Schiff base deprotonation during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8580–8584. doi: 10.1073/pnas.83.22.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duñach M., Seigneuret M., Rigaud J. L., Padrós E. Influence of cations on the blue to purple transition of bacteriorhodopsin. Comparison of Ca2+ and Hg2+ binding and their effect on the surface potential. J Biol Chem. 1988 Nov 25;263(33):17378–17384. [PubMed] [Google Scholar]
- Eisfeld W., Pusch C., Diller R., Lohrmann R., Stockburger M. Resonance Raman and optical transient studies on the light-induced proton pump of bacteriorhodopsin reveal parallel photocycles. Biochemistry. 1993 Jul 20;32(28):7196–7215. doi: 10.1021/bi00079a017. [DOI] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Bacteriorhodopsin in ice. Accelerated proton transfer from the purple membrane surface. FEBS Lett. 1990 Dec 17;277(1-2):277–280. doi: 10.1016/0014-5793(90)80864-f. [DOI] [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Surface-bound optical probes monitor protein translocation and surface potential changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5996–6000. doi: 10.1073/pnas.89.13.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle J., Oesterhelt D., Dencher N. A. Decoupling of photo- and proton cycle in the Asp85-->Glu mutant of bacteriorhodopsin. EMBO J. 1993 Oct;12(10):3721–3727. doi: 10.1002/j.1460-2075.1993.tb06049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W., Logunov I., Schulten K., Sheves M. Molecular dynamics study of bacteriorhodopsin and artificial pigments. Biochemistry. 1994 Mar 29;33(12):3668–3678. doi: 10.1021/bi00178a025. [DOI] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966 Aug 27;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Jencks W. P. The utilization of binding energy in coupled vectorial processes. Adv Enzymol Relat Areas Mol Biol. 1980;51:75–106. doi: 10.1002/9780470122969.ch2. [DOI] [PubMed] [Google Scholar]
- Jonas R., Koutalos Y., Ebrey T. G. Purple membrane: surface charge density and the multiple effect of pH and cations. Photochem Photobiol. 1990 Dec;52(6):1163–1177. doi: 10.1111/j.1751-1097.1990.tb08455.x. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Kamikubo H., Tokunaga F., Brown L. S., Yamazaki Y., Maeda A., Sheves M., Needleman R., Lanyi J. K. Energy coupling in an ion pump. The reprotonation switch of bacteriorhodopsin. J Mol Biol. 1994 Nov 4;243(4):621–638. doi: 10.1016/0022-2836(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Koch M. H., Dencher N. A., Oesterhelt D., Plöhn H. J., Rapp G., Büldt G. Time-resolved X-ray diffraction study of structural changes associated with the photocycle of bacteriorhodopsin. EMBO J. 1991 Mar;10(3):521–526. doi: 10.1002/j.1460-2075.1991.tb07978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., Misra S., Ebrey T. G. pH dependence of light-induced proton release by bacteriorhodopsin. FEBS Lett. 1993 Sep 27;331(1-2):31–34. doi: 10.1016/0014-5793(93)80291-2. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Sherman W. V., Caplan S. R. Kinetic isotope effects in the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1976 Dec 22;2(3):267–276. doi: 10.1007/BF00535372. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Tittor J., Váró G., Krippahl G., Oesterhelt D. Influence of the size and protonation state of acidic residue 85 on the absorption spectrum and photoreaction of the bacteriorhodopsin chromophore. Biochim Biophys Acta. 1992 Jan 30;1099(1):102–110. [PubMed] [Google Scholar]
- Liu S. Y., Govindjee R., Ebrey T. G. Light-induced currents from oriented purple membrane: II. Proton and cation contributions to the photocurrent. Biophys J. 1990 May;57(5):951–963. doi: 10.1016/S0006-3495(90)82615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Y. Light-induced currents from oriented purple membrane: I. Correlation of the microsecond component (B2) with the L-M photocycle transition. Biophys J. 1990 May;57(5):943–950. doi: 10.1016/S0006-3495(90)82614-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Nakasako M., Kataoka M., Amemiya Y., Tokunaga F. Crystallographic characterization by X-ray diffraction of the M-intermediate from the photo-cycle of bacteriorhodopsin at room temperature. FEBS Lett. 1991 Nov 4;292(1-2):73–75. doi: 10.1016/0014-5793(91)80837-s. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J., Bamberg E. A unifying concept for ion translocation by retinal proteins. J Bioenerg Biomembr. 1992 Apr;24(2):181–191. doi: 10.1007/BF00762676. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J. FTIR difference spectroscopy of bacteriorhodopsin: toward a molecular model. J Bioenerg Biomembr. 1992 Apr;24(2):147–167. doi: 10.1007/BF00762674. [DOI] [PubMed] [Google Scholar]
- Sampogna R. V., Honig B. Environmental effects on the protonation states of active site residues in bacteriorhodopsin. Biophys J. 1994 May;66(5):1341–1352. doi: 10.1016/S0006-3495(94)80925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvignier G., Gerwert K. Proton uptake mechanism of bacteriorhodopsin as determined by time-resolved stroboscopic-FTIR-spectroscopy. Biophys J. 1992 Nov;63(5):1393–1405. doi: 10.1016/S0006-3495(92)81722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Gerstein M., Oesterhelt D., Henderson R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 1993 Jan;12(1):1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Surface pH controls purple-to-blue transition of bacteriorhodopsin. A theoretical model of purple membrane surface. Biophys J. 1989 Aug;56(2):369–383. doi: 10.1016/S0006-3495(89)82683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Mechanism of free energy coupling in active transport. Annu Rev Biochem. 1983;52:379–409. doi: 10.1146/annurev.bi.52.070183.002115. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson T. E., Milder S. J., Miercke L. J., Betlach M. C., Shand R. F., Stroud R. M., Kliger D. S. Effects of Asp-96----Asn, Asp-85----Asn, and Arg-82----Gln single-site substitutions on the photocycle of bacteriorhodopsin. Biochemistry. 1991 Sep 24;30(38):9133–9142. doi: 10.1021/bi00102a003. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Kinetic and spectroscopic evidence for an irreversible step between deprotonation and reprotonation of the Schiff base in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5008–5015. doi: 10.1021/bi00234a024. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Pathways of the rise and decay of the M photointermediate(s) of bacteriorhodopsin. Biochemistry. 1990 Mar 6;29(9):2241–2250. doi: 10.1021/bi00461a006. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Cao Y., Chang M., Ni B., Needleman R., Lanyi J. K. The two consecutive M substates in the photocycle of bacteriorhodopsin are affected specifically by the D85N and D96N residue replacements. Photochem Photobiol. 1992 Dec;56(6):1049–1055. doi: 10.1111/j.1751-1097.1992.tb09728.x. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Váró G., Chang M., Ni B., Needleman R., Lanyi J. K. Pathways of proton release in the bacteriorhodopsin photocycle. Biochemistry. 1992 Sep 15;31(36):8535–8543. doi: 10.1021/bi00151a022. [DOI] [PubMed] [Google Scholar]