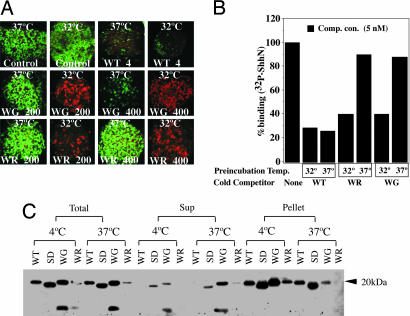
Fig. 2.
Temperature dependence of WG and WR protein activities. (A) Neural plate signaling of WG and WR variants. Neural plate explants were incubated at 37°C and 32°C at the indicated concentrations of ShhN (in nM) and stained to monitor expression of Pax-7 (green; control panels) and HNF3β (red). Stimulation with WT protein (4 nM) suppressed Pax-7 expression at both temperatures, whereas variant proteins at 37°C failed to suppress Pax-7 at 200 nM protein concentrations (WG and WR) or at 400 nM protein concentrations (WR). However, at 32°C, WG and WR proteins at 200 and 400 nM suppressed Pax-7 (data not shown) and induced HNF3-b expression (red). (B) Ptch-binding of WG and WR variants. Proteins were preincubated at 37°C or 32°C for 1 h, then added (5 nM) to EcR-293 cells stably expressing Ptc-CTD in the presence of 32P-ShhN. WG and WR variants failed to compete with 32P-ShhN when preincubated at 37°C, but showed some activity when preincubated at 32°C. (C) Immunoprecipitation of variant proteins by 5E1 monoclonal antibody. WT ShhN and surface D (SD) (15), WG, and WR variants were all immunoprecipitated by 5E1-monoclonal antibody when preincubated at 4°C (bulk of proteins found in pellet, not supernatant), but after preincubation at 37°C, WG and WR were predominantly found in the supernatant. Proteins were detected by immunoblotting with anti-ShhN polyclonal antibodies.