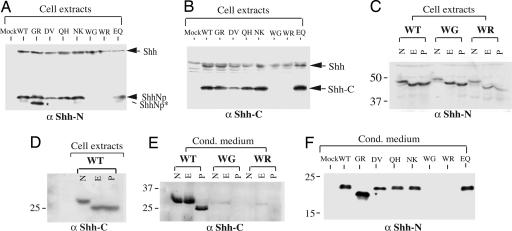
Fig. 3.
Effect of HPE mutations on Shh protein processing. (A and B) Western blot analysis of cell extracts from HEK-293 cells transiently transfected with full-length WT and variant Shh constructs. Anti-ShhN polyclonal antibodies detected the full-length Shh protein (45 kDa, indicated by arrow), the processed and lipid-modified N-terminal fragment (ShhNp, indicated by arrowhead), and an abnormal processed N-terminal fragment (ShhNp*, indicated by star) generated from the GR variant (A), whereas anti-ShhC antibodies detected full-length Shh and the ShhC fragment (B). The WG and WR variants produced neither ShhNp nor ShhC fragments. (C-E) Glycosidase digestion. Extracts (C and D) or conditioned medium (E) from HEK-293 cells transfected with Shh constructs were immunoblotted after denaturation and no further treatment (N) or treatment with Endo H (E) or PNGase F (P). Proteins were separated in 10% (C) or 15% (D and E) SDS/PAGE and immunoblotted with anti-ShhN (C) or anti-ShhC (D and E) antibodies. (F) Conditioned medium from transfected HEK-293 cells was immunoblotted and analyzed with anti-ShhN antibodies. Note the greater abundance of the abnormally processed ShhN GR variant in conditioned medium.