Fig. 4.
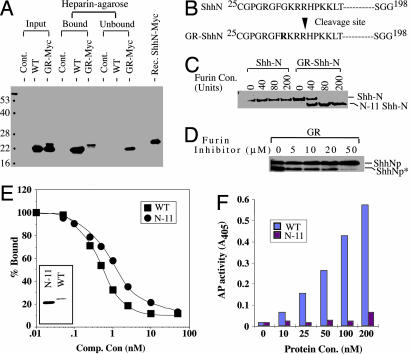
Abnormal processing and functional characterization of ShhN from the GR variant. (A) Heparin-agarose binding. Conditioned media from HEK-293 cells transfected with ShhN or GR-ShhN-Myc were separated with heparin-agarose and immunoblotted with anti-ShhN polyclonal antibodies. The C-terminal Myc epitope can be detected in both the slower- and faster-migrating forms of GR-ShhN-Myc (data not shown), indicating that the abnormal truncation occurs at the N terminus. WT ShhN and the longer form of GR-ShhN-Myc are both retained by heparin agarose, but the truncated form of the GR variant is not. Note the comigration of slower-migrating GR-ShhN-Myc and recombinant ShhN-Myc protein purified from E. coli.(B) Determination of cleavage site. The abnormally truncated ShhN*-Myc protein was purified by lack of binding to heparin-agarose and with the monoclonal antibody 5E1 and subjected to N-terminal sequence analysis by Edman degradation. This sequence indicated that cleavage occurred between Arg (35) and His (36) residues. (C and D) Furin cleavage of recombinant GR-ShhN protein in vitro and in vivo. (C) Purified, recombinant ShhN (1.5 μg) and GR-ShhN (2.0 μg) proteins treated with 0, 40, 80, and 200 units of furin protease (Sigma) were analyzed by SDS/PAGE and Coomassie blue staining. (D) EcR-293 cells with stably integrated construct for expression of GR-Shh full-length protein were induced with ponasterone in the presence of 0, 5, 10, 20, and 50 μM of furin inhibitor I, and cell extracts were analyzed by immunoblotting with anti-ShhN antibodies. (E and F) Ptc-binding and Shh signaling activities of recombinant ShhN*. (E) Recombinant WT ShhN and protein lacking 11 N-terminal residues (N-11) proteins were purified (Inset) and used as competitor for 32P-ShhN binding to EcR-293 cells expressing Ptc-CTD. (F) Signaling activities of ShhN WT and N-11 in C3H10T1/2 cells.