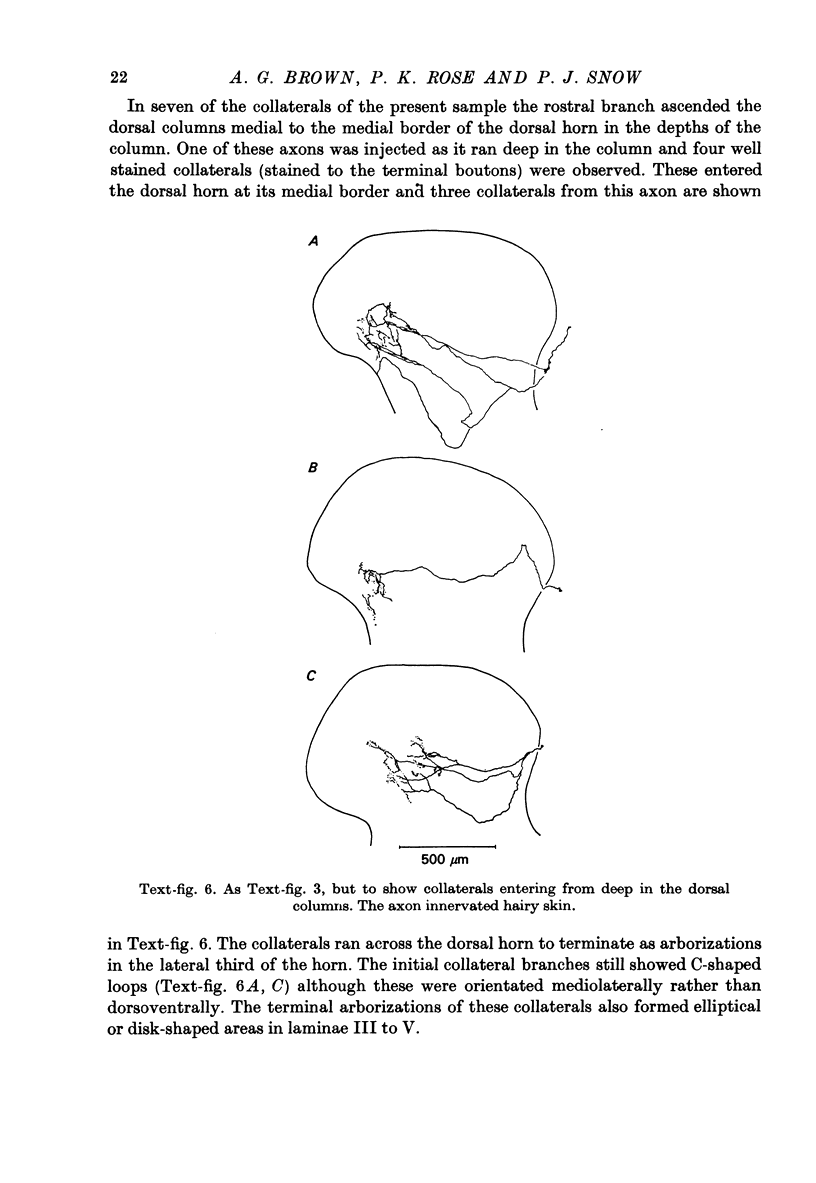
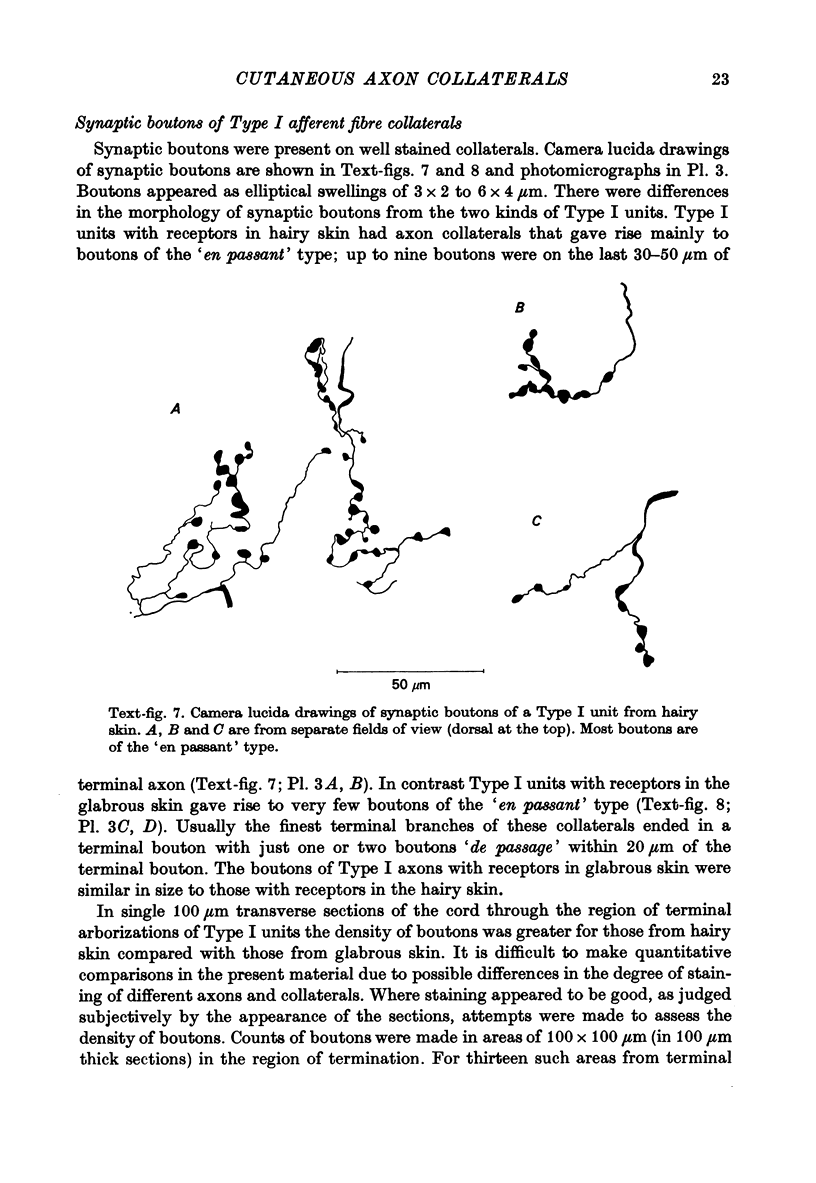
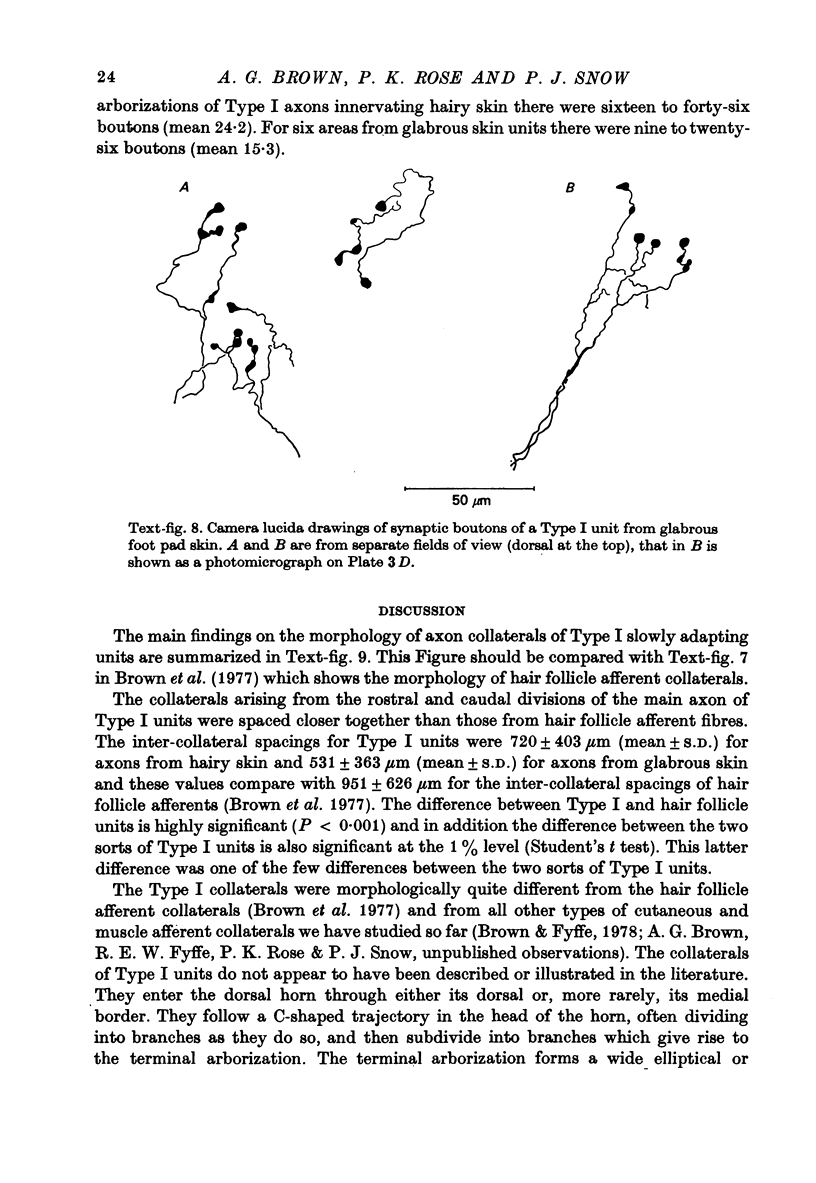
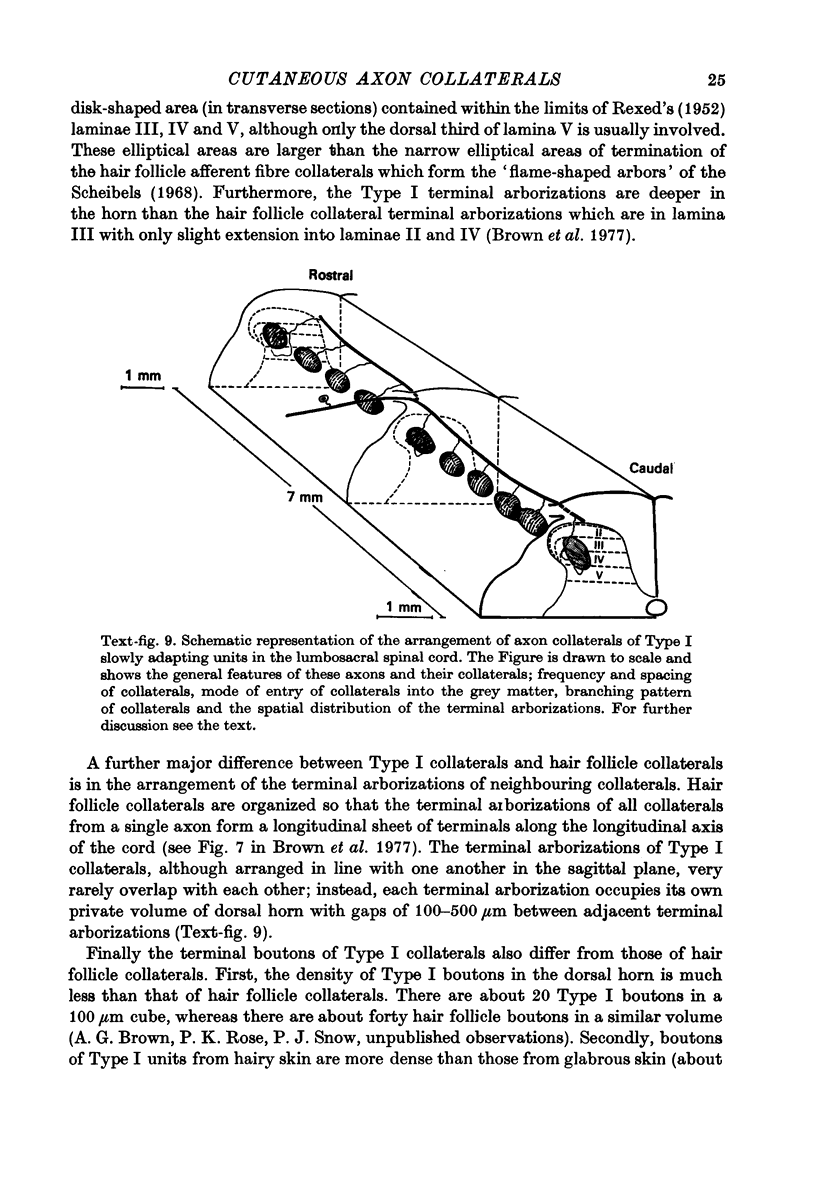
Abstract
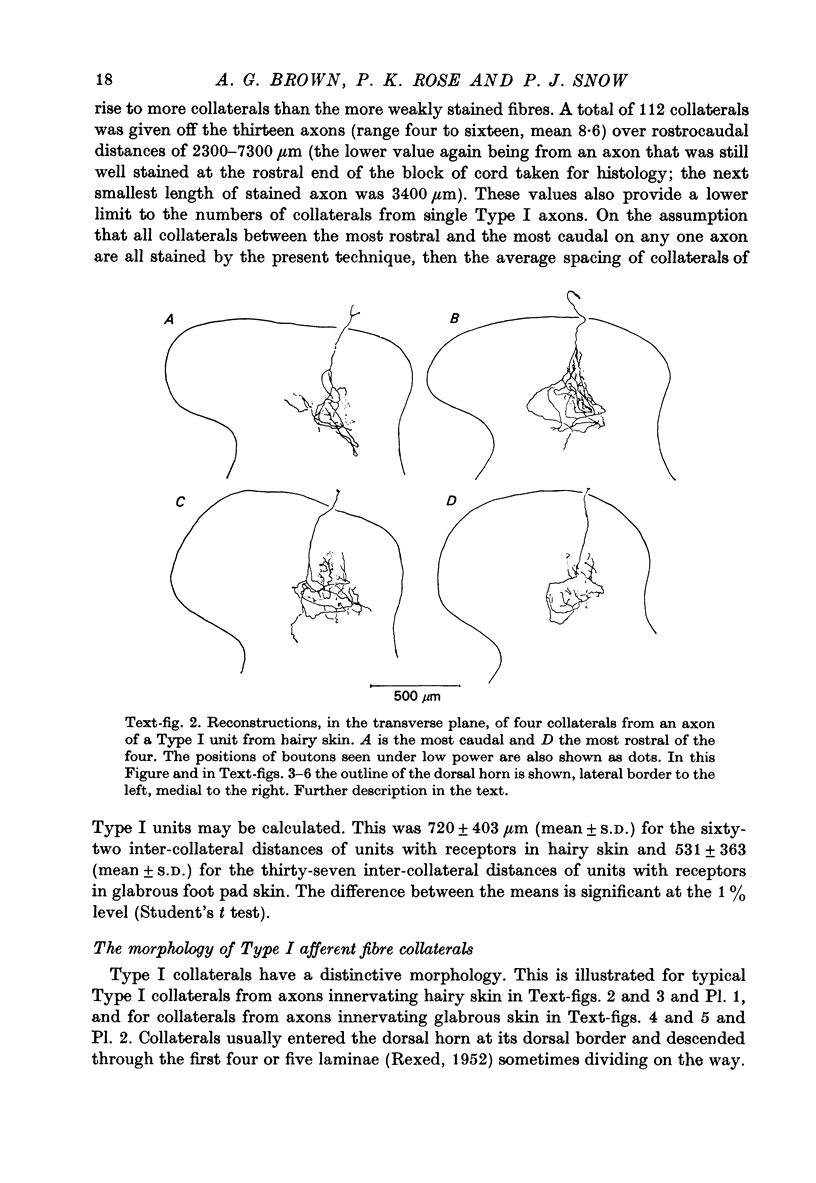
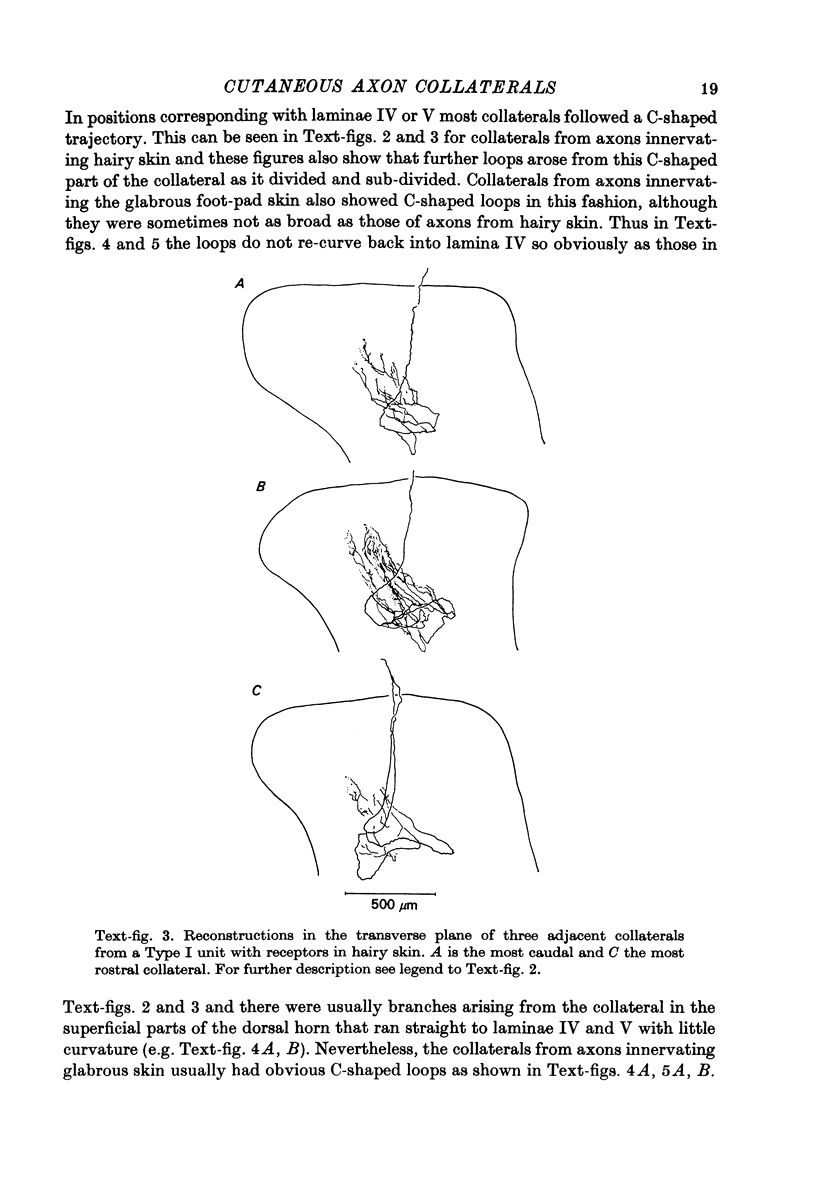
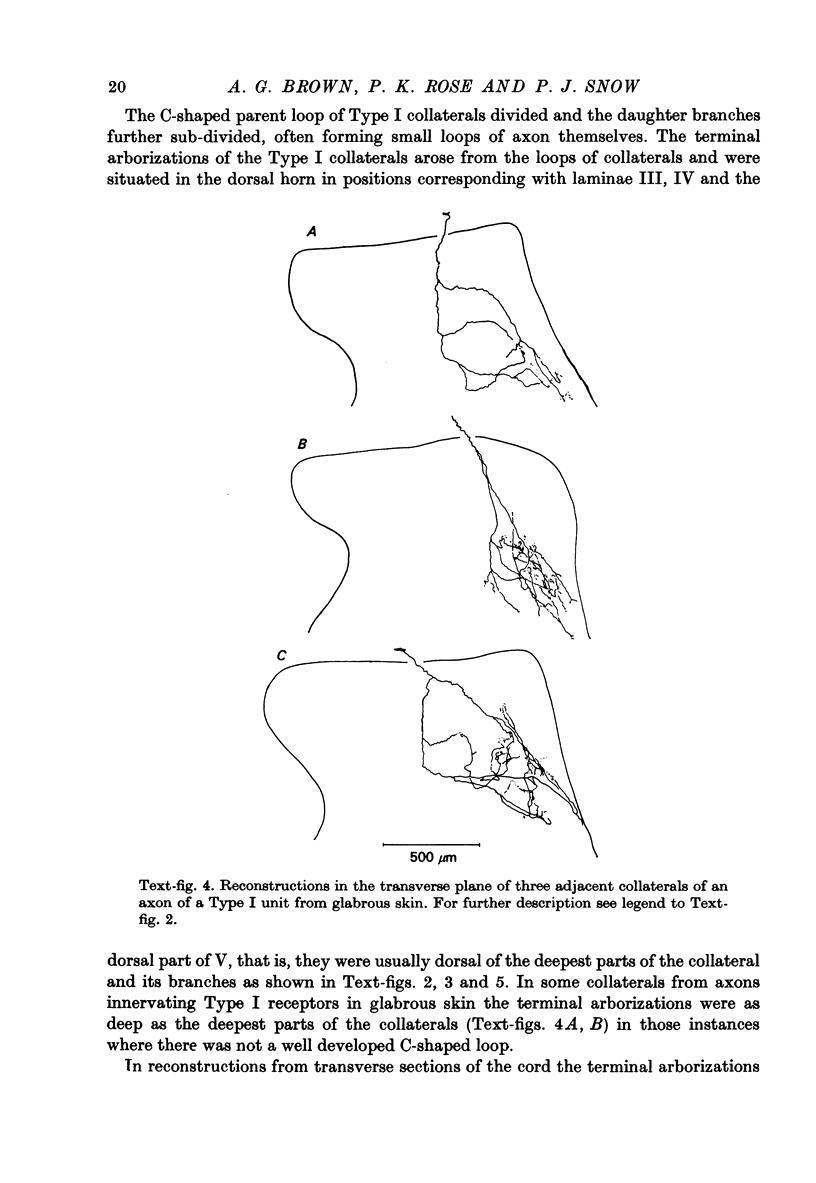
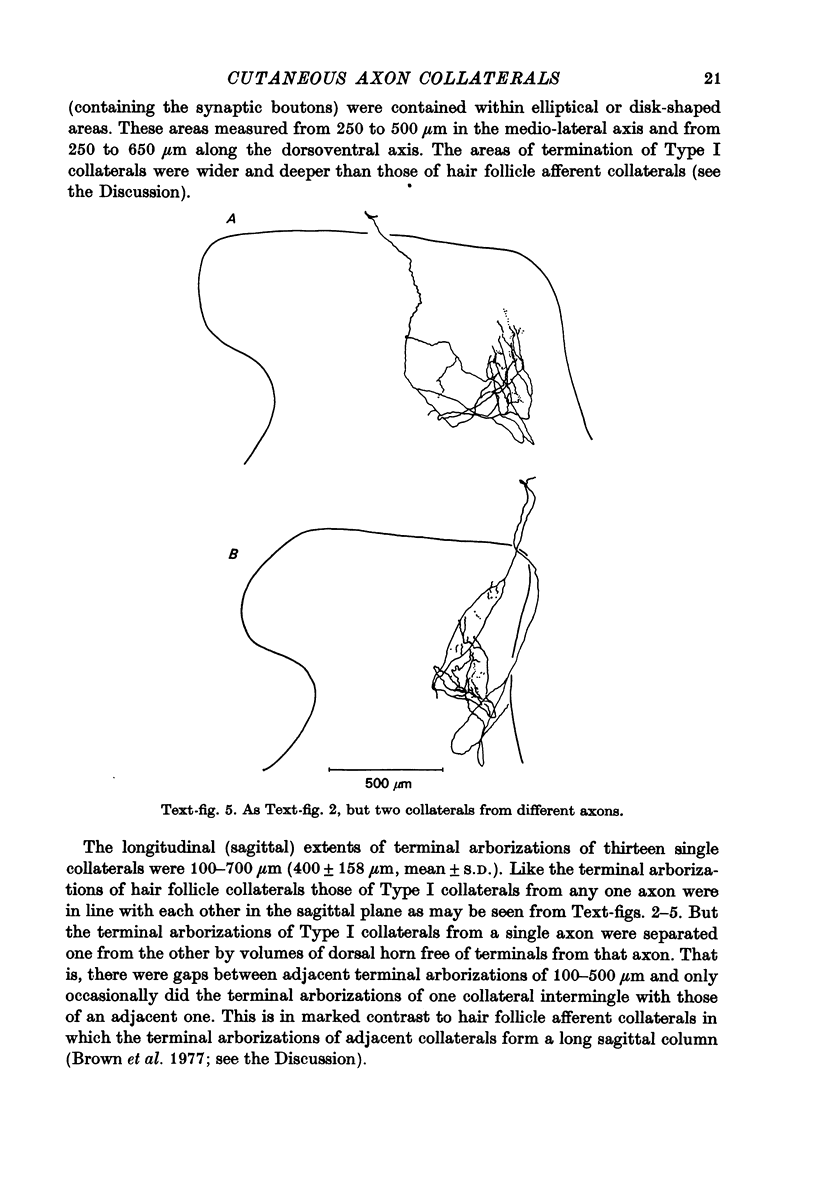
1. The morphology of the collaterals of single axons innervating Type I slowly adapting receptors was studied by using the intra-axonal injection of the enzyme horseradish peroxidase in anaesthetized cats. The axons were impaled near their dorsal root entrance zone in the lumbosacral cord. The morphology was revealed by subsequent histochemistry. 2. Thirteen Type I axons were stained, nine with receptors in the hairy skin and four with receptors in the glabrous foot pad skin. Twelve axons could be traced back into their dorsal roots and 11 of these divided into rostral and caudal branches shortly after entering the spinal cord. 3. One hundred and twelve collaterals were given off the thirteen axons and all well filled collaterals had a similar morphology. In the dorsal horn they gave rise to wide elliptical areas of terminal arborization (in transverse sections of cord) that were limited to laminae III, IV and the dorsal part of lamina V. The terminal arborizations of collaterals from the same axon were in line in the saggittal plane, but only rarely did the terminal arborizations of adjacent collaterals overlap; usually there was a gap between adjacent terminal arborizations. 4. Synaptic boutons of Type I units from hairy skin were mainly of the "en passant" variety whereas those of Type I units from glabrous skin were generally "boutons terminaux" with very few boutons "de passage". 5. The morphology of axon collaterals of Type I units is compared with that of hair follicle units.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Brown A. G. Cutaneous axons and sensory neurones in the spinal cord. Br Med Bull. 1977 May;33(2):109–112. doi: 10.1093/oxfordjournals.bmb.a071409. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1977 Nov;272(3):779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. B., Moraff H., Tapper D. N. Functional organization of the cat's dorsal horn: spontaneous activity and central cell response to single impulses in single type I fibers. J Neurophysiol. 1973 Sep;36(5):827–839. doi: 10.1152/jn.1973.36.5.827. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Petit D., Warren R. M. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Devor M. A microelectrophoretic delivery technique for use with horseradish peroxidase. Brain Res. 1974 Mar 15;68(1):167–173. doi: 10.1016/0006-8993(74)90541-1. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971 May 7;28(2):217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- Mann M. D. Axons of dorsal spinocerebellar tract which respond to activity in cutaneous receptors. J Neurophysiol. 1971 Nov;34(6):1035–1050. doi: 10.1152/jn.1971.34.6.1035. [DOI] [PubMed] [Google Scholar]
- Mann M. D., Kasprzak H., Tapper D. N. Ascending dorsolateral pathways relaying type I afferent activity. Brain Res. 1971 Mar 19;27(1):176–178. doi: 10.1016/0006-8993(71)90380-5. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Res. 1968 Jun;9(1):32–58. doi: 10.1016/0006-8993(68)90256-4. [DOI] [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Brown P. B., Moraff H. Functional organization of the cat's dorsal horn: connectivity of myelinated fiber systems of hairy skin. J Neurophysiol. 1973 Sep;36(5):817–826. doi: 10.1152/jn.1973.36.5.817. [DOI] [PubMed] [Google Scholar]
- Tapper D. N. Stimulus-response relationships in the cutaneous slowly-adapting mechanoreceptor in hairy skin of the cat. Exp Neurol. 1965 Dec;13(4):364–385. doi: 10.1016/0014-4886(65)90125-1. [DOI] [PubMed] [Google Scholar]





