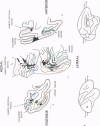
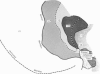
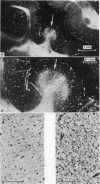
Abstract
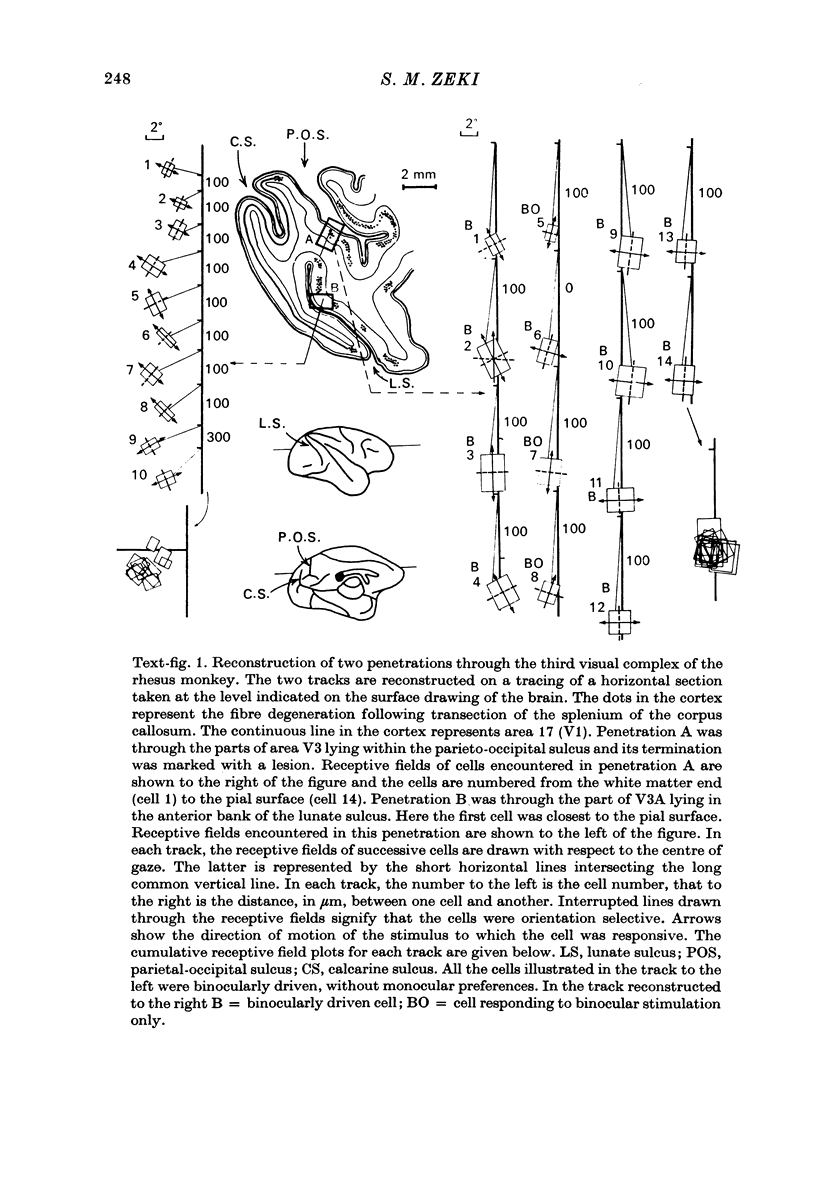
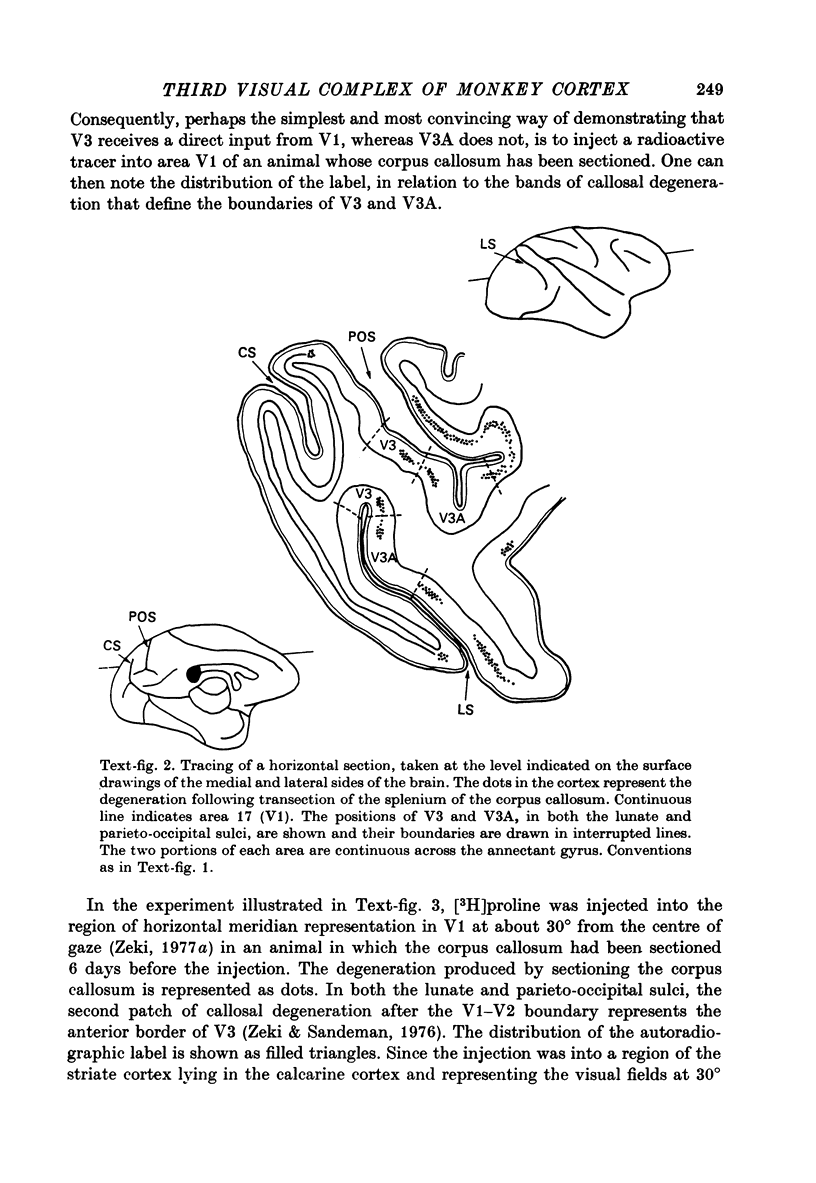
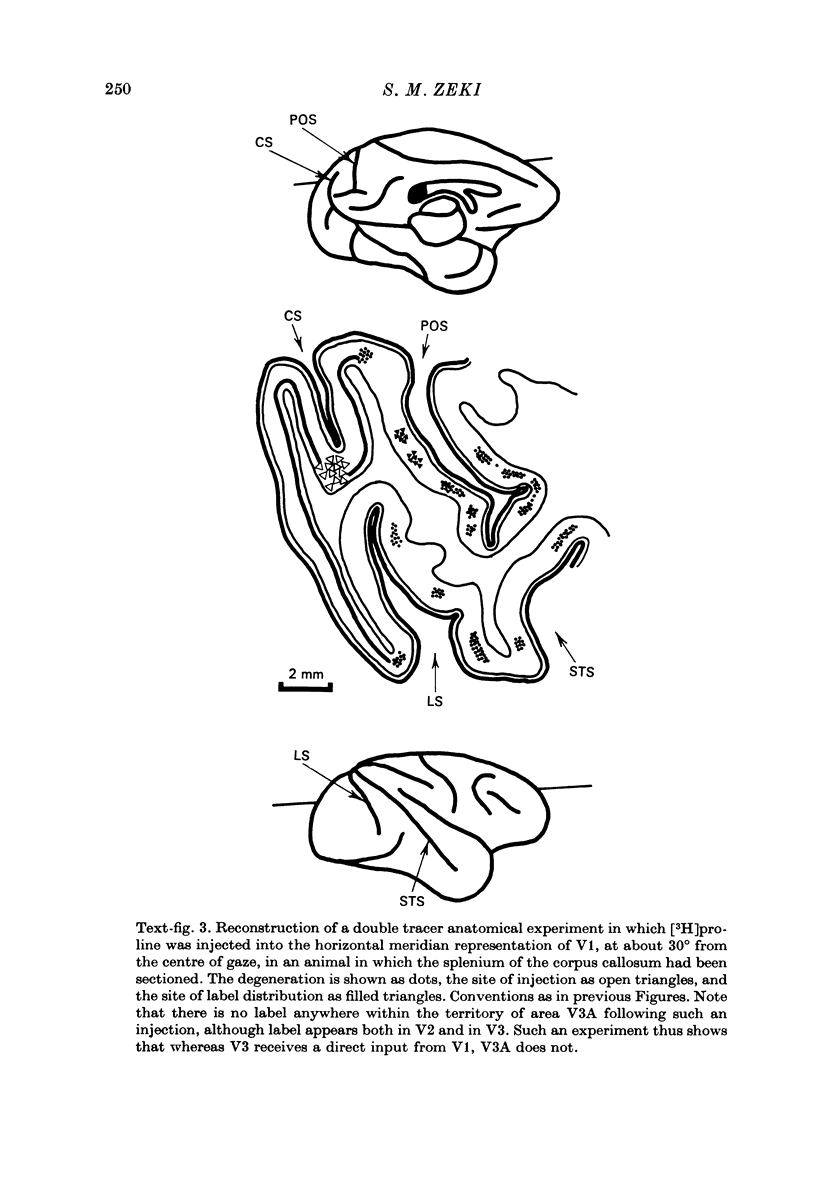
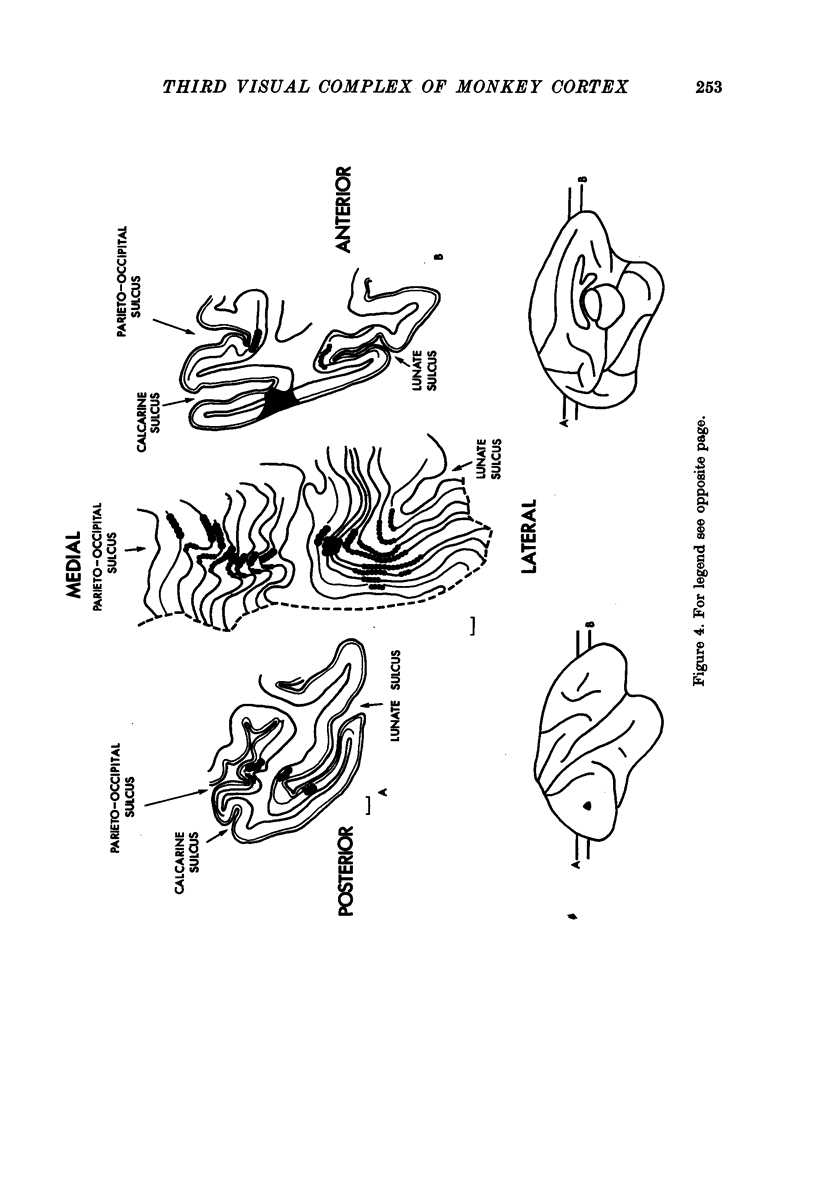
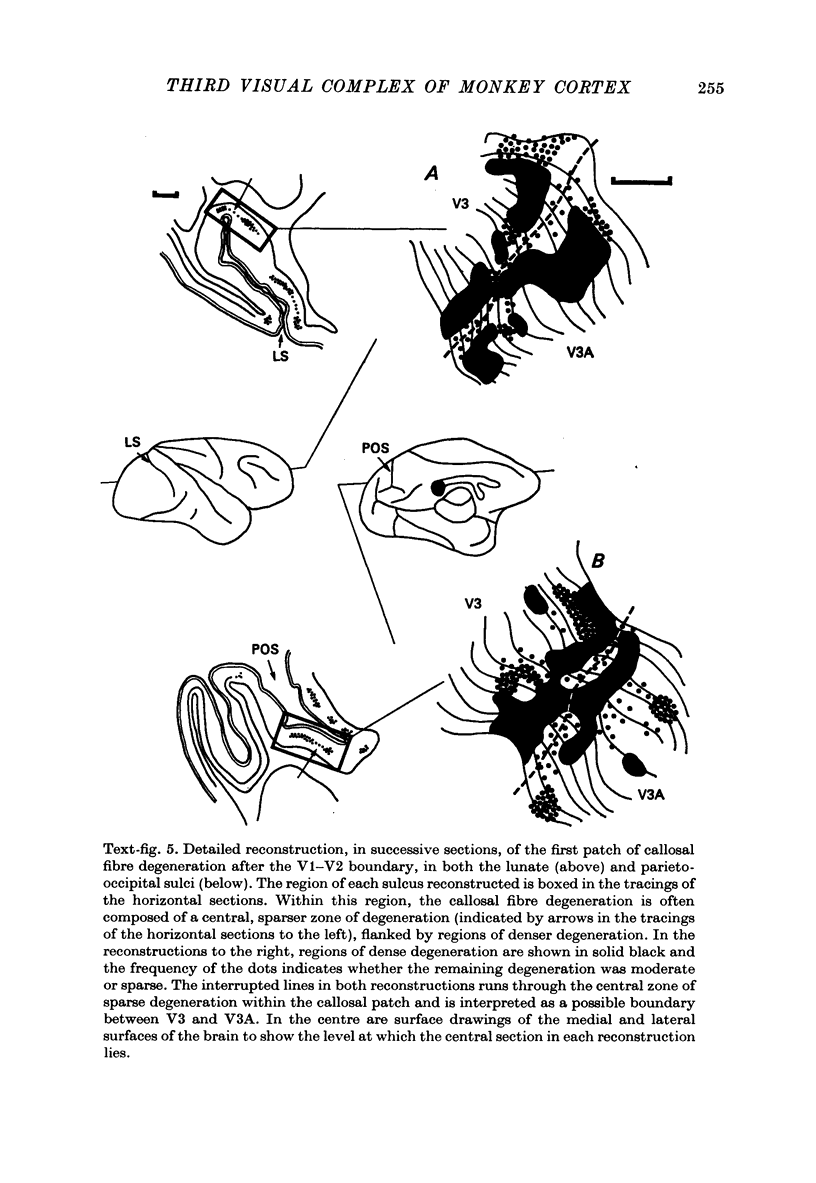
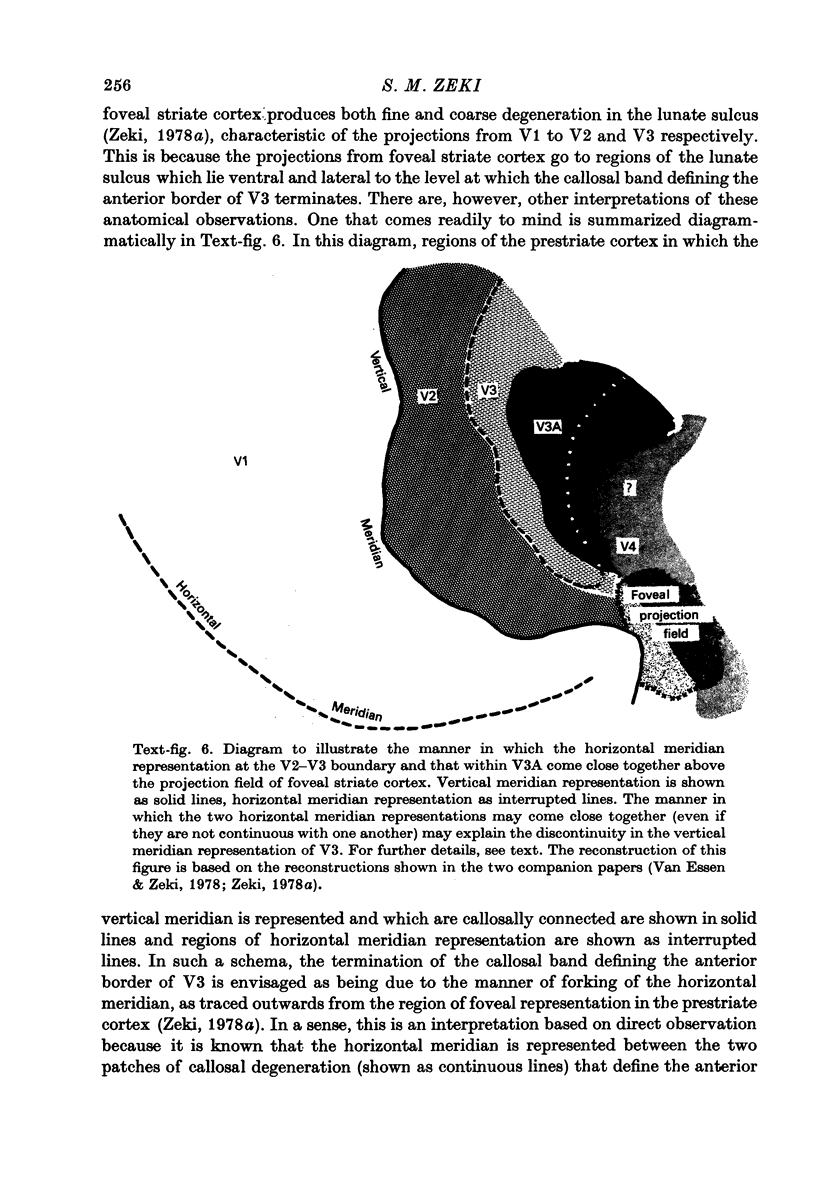
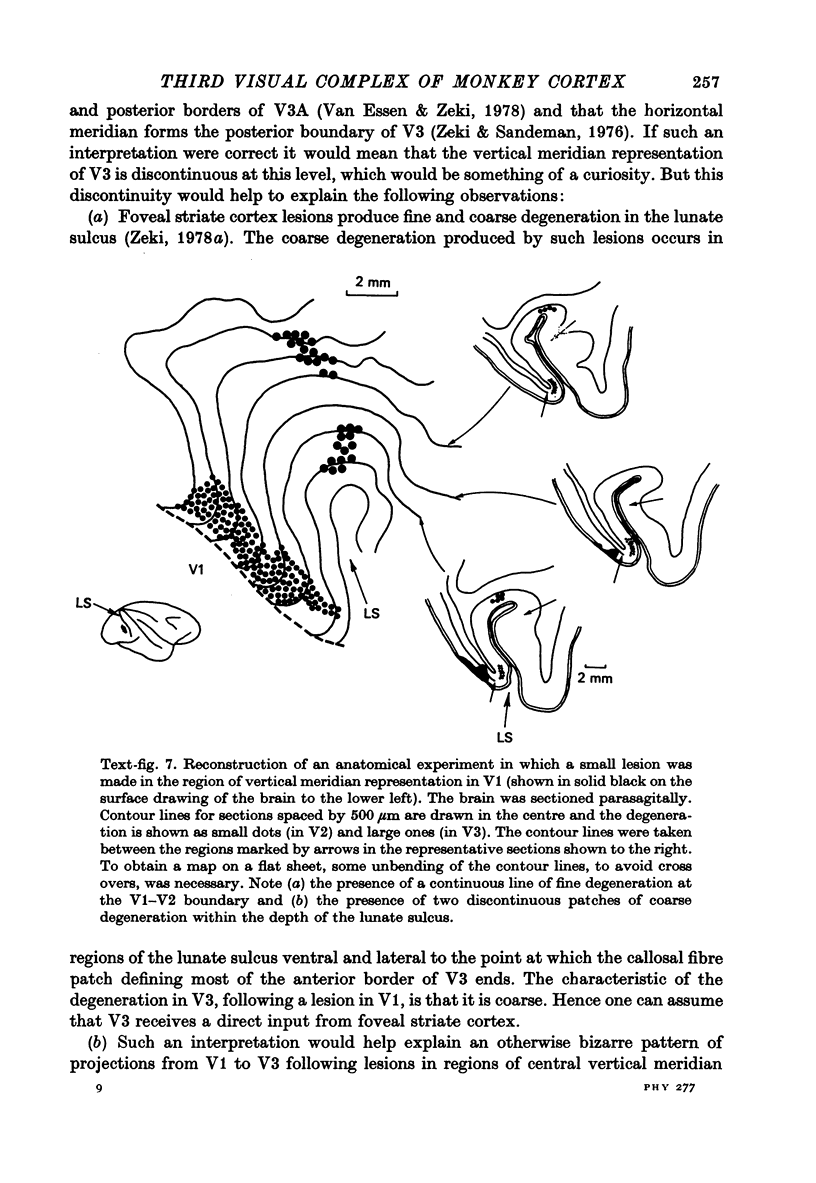
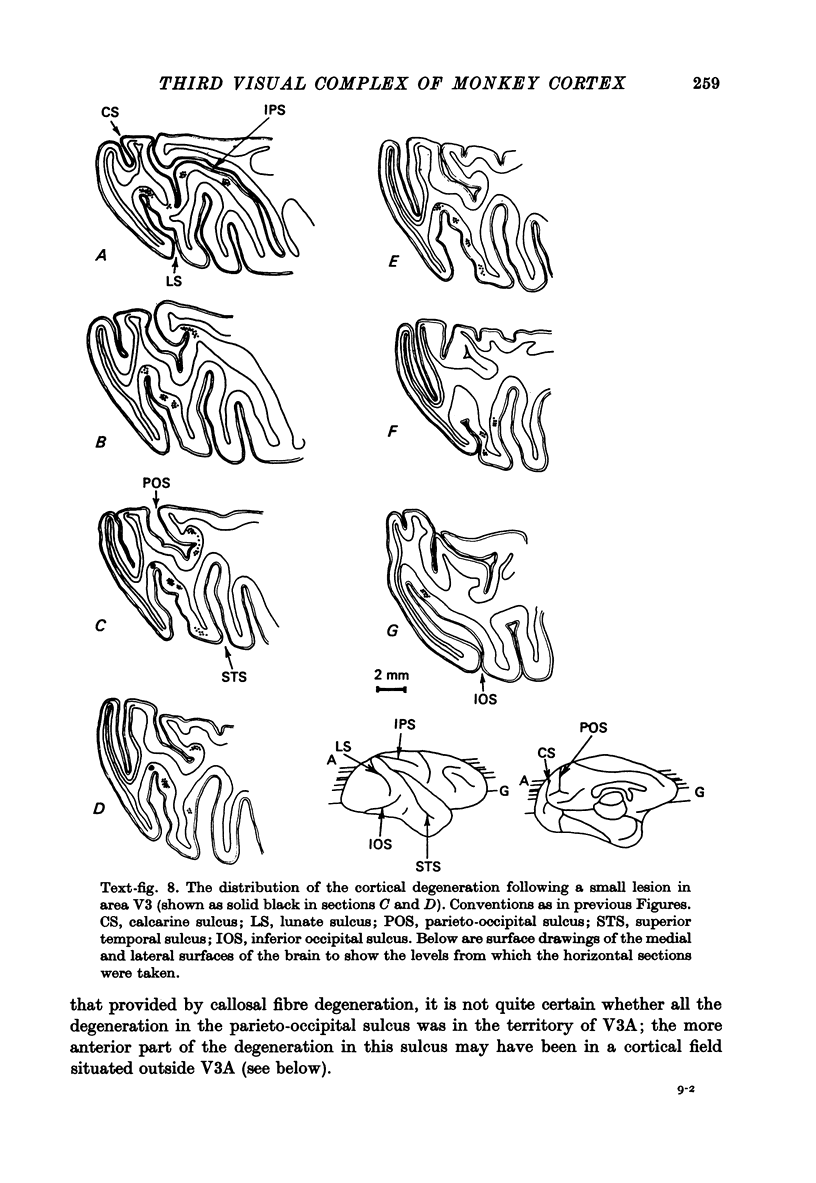
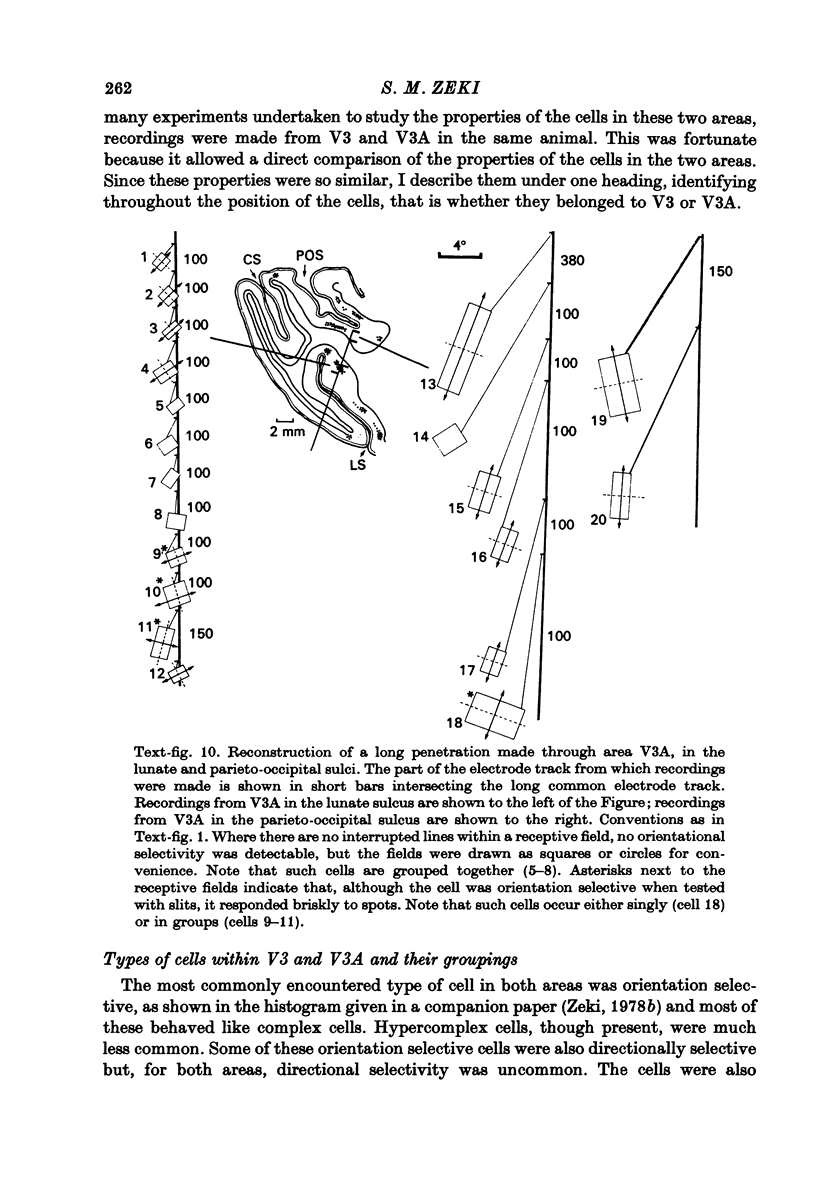
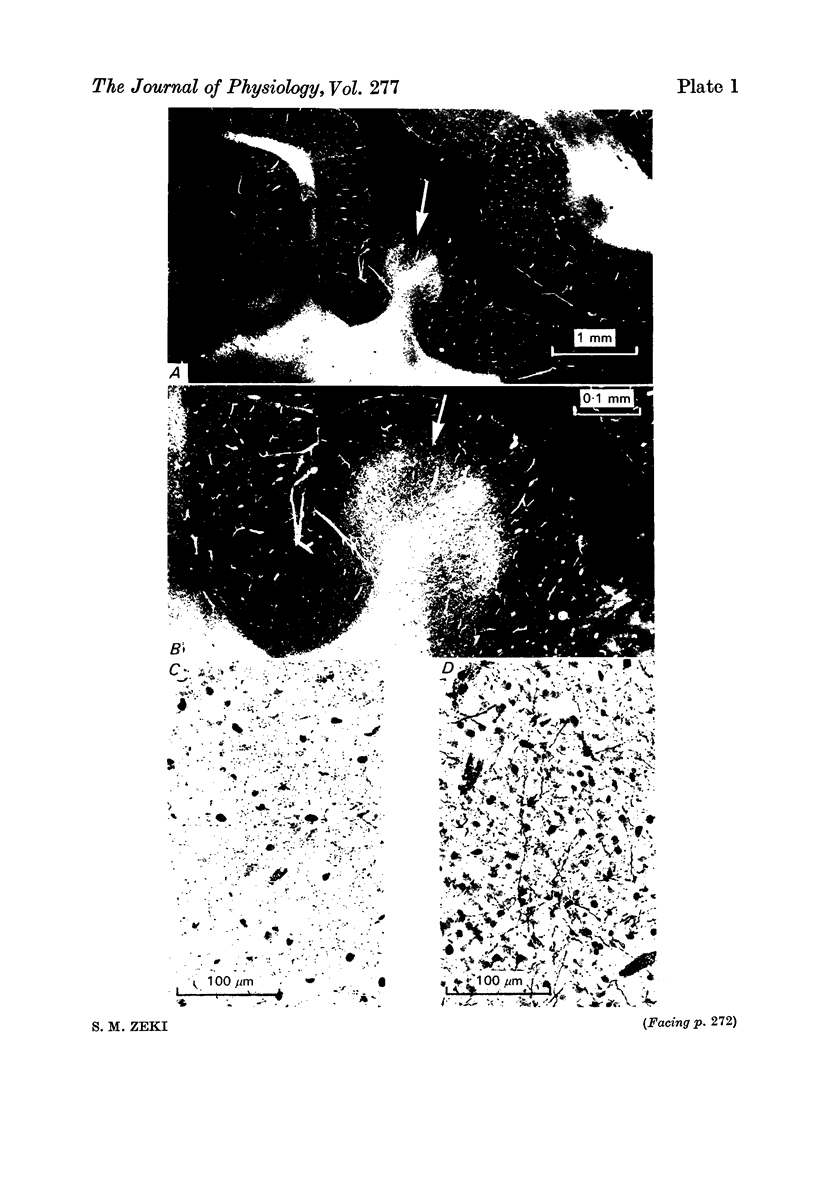
1. Two independent but neighbouring visual areas, V3 and V3A, sharing a common cytoarchitectural plan, but in each one of which the visual fields are separately represented, have been studied anatomically, functionally, and in combined anatomico-physiological experiments. 2. The properties of single cells in the two areas are so similar, judged by the techniques used in this study, that it is often impossible to tell whether any one penetration was sampling from cells in V3 or V3A. This is especially so if the cells have receptive fields in the lower hemi-quadrants, since the vertical meridian of the lower visual fields is represented along the V3-V3A boundary and since a transition from V3 to V3A along this border is not accompanied by a shift in receptive field positions of cells. 3. Since the visual fields, including the vertical meridian, are separately represented in these two areas, and since regions of vertical meridian representation are callosally connected, a simple and certain method of specifying the boundary between V3 and V3A is to examine the degeneration following section of the callosal splenium. A heavy patch of degeneration then marks the V3-V3A boundary. Within this patch, however, is a sub-patch containing fewer callosal fibres, or none at all. The boundary between V3 and V3A was taken to be at this subpatch. 4. Since the horizontal meridian is represented at the V2-V3 boundary, and since V1 projects to both these areas, sending coarse fibres to V3 and fine fibres to V2, it was found that the boundary between V2 and V3 could be precisely drawn by making a lesion in the horizontal meridian representation in V1 and noting where, in the prestriate cortex, fine fibres give way to coarse ones, without an intervening gap. 5. Double tracer anatomical experiments, in which tritiated proline was injected into V1 of animals whose callosal splenium had been sectioned, showed that whereas V3 receives a direct input from V1, V3A does not. V3A, instead, was found to receive an input from V3. Double tracer anatomical experiments were undertaken to study a possible input from V2 to V3A. Although such experiments did not reveal a direct input from V2 to V3A, they were not entirely conclusive. 6. The vast majority of cells in V3 and V3A were binocularly driven, without obvious monocular preferences. Some cells, however, though responding to stimulation of the individual eyes, summated their responses to binocular stimulation. Others responded only when both eyes were simulataneously stimulated. In any oblique penetration, cells preferring binocular stimulation only occurred either singly or in groups. 7. In an oblique penetration, the shift from a cell responding to binocular stimulation only to one responding equally well to stimulation of either eye was not necessarily accompanied by a shift in orientational preferences, shifts in the former...
Full text
PDF








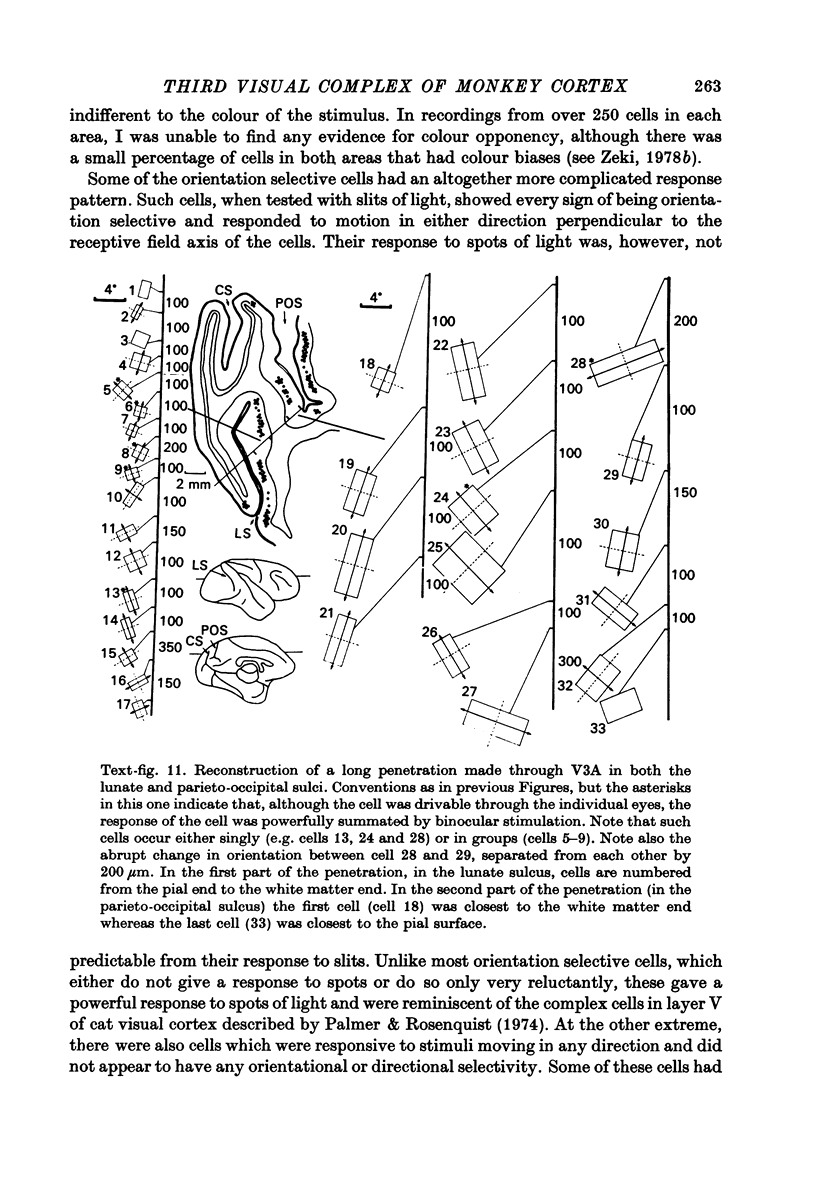

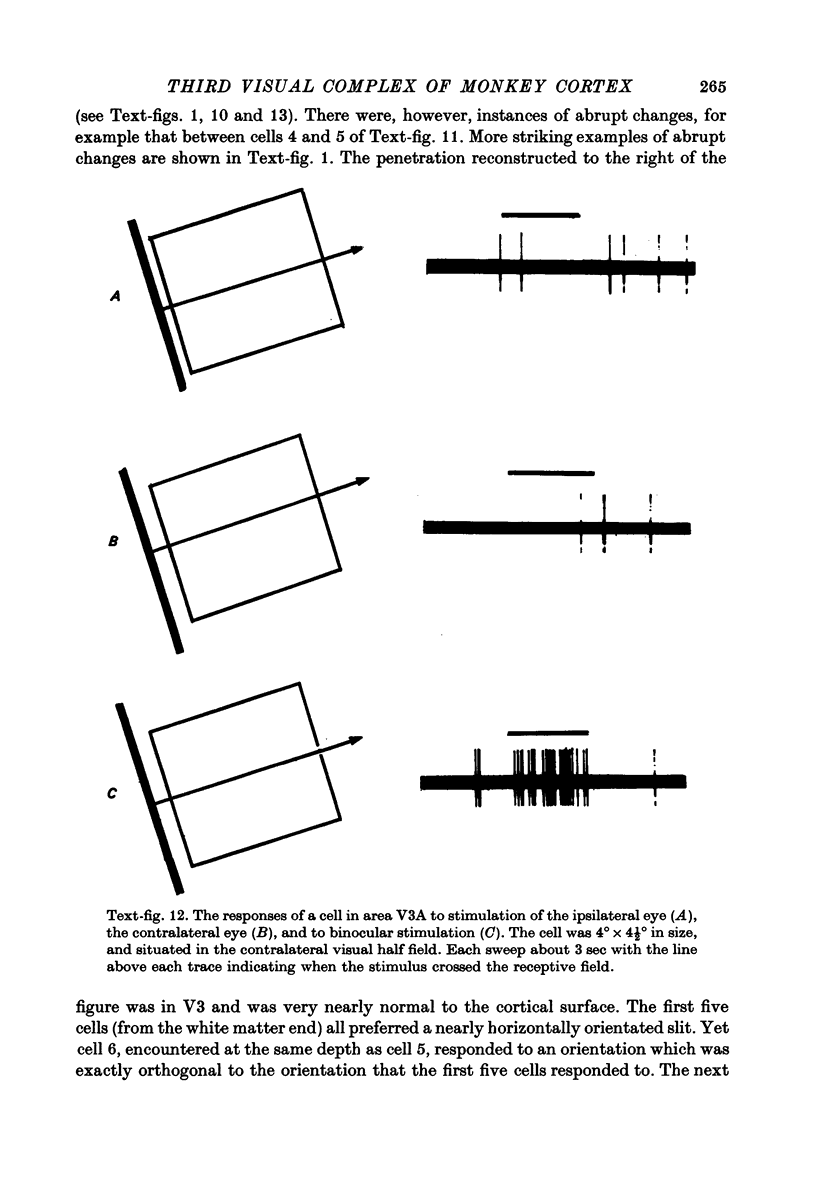
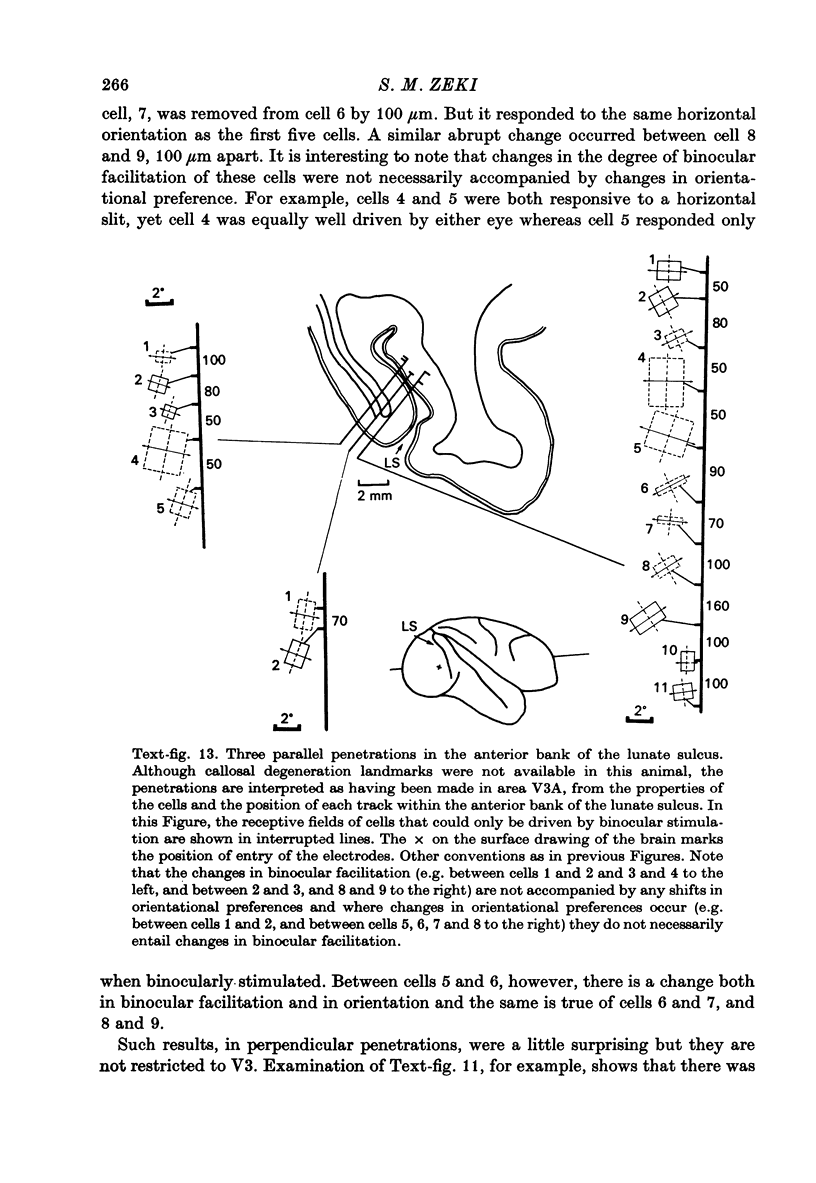
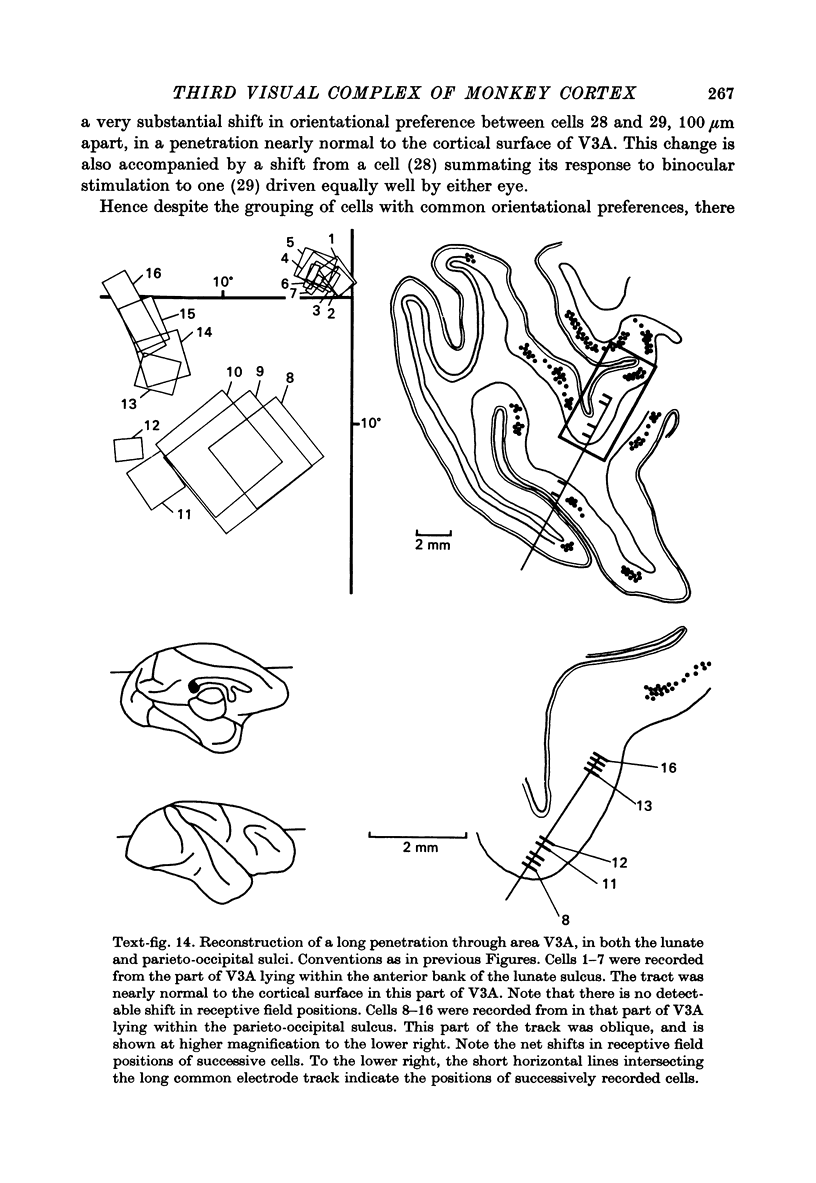





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allman J. M., Kaas J. H. The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus). Brain Res. 1975 Dec 26;100(3):473–487. doi: 10.1016/0006-8993(75)90153-5. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 1969 Jul;9(7):733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- DANIEL P. M., WHITTERIDGE D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961 Dec;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen D. C., Zeki S. M. The topographic organization of rhesus monkey prestriate cortex. J Physiol. 1978 Apr;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Stereoscopic vision in macaque monkey. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature. 1970 Jan 3;225(5227):41–42. doi: 10.1038/225041a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Uniformity of monkey striate cortex: a parallel relationship between field size, scatter, and magnification factor. J Comp Neurol. 1974 Dec 1;158(3):295–305. doi: 10.1002/cne.901580305. [DOI] [PubMed] [Google Scholar]
- Martinez-Millán L., Holländer H. Cortico-cortical projections from striate cortex of the squirrel monkey (Saimiri sciureus). A radioautographic study. Brain Res. 1975 Jan 17;83(3):405–417. doi: 10.1016/0006-8993(75)90833-1. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Spatz W. B., Tigges J., Tigges M. Subcortical projections, cortical associations, and some intrinsic interlaminar connections of the striate cortex in the squirrel monkey (Saimiri). J Comp Neurol. 1970 Oct;140(2):155–174. doi: 10.1002/cne.901400203. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Cortical projections from two prestriate areas in the monkey. Brain Res. 1971 Nov;34(1):19–35. doi: 10.1016/0006-8993(71)90348-9. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol. 1974 Feb;236(3):549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. M. Interhemispheric connections of prestriate cortex in monkey. Brain Res. 1970 Apr 1;19(1):63–75. doi: 10.1016/0006-8993(70)90237-4. [DOI] [PubMed] [Google Scholar]
- Zeki S. M., Sandeman D. R. Combined anatomical and electrophysiological studies on the boundary between the second and third visual areas of rhesus monkey cortex. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):555–562. doi: 10.1098/rspb.1976.0094. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. The cortical projections of foveal striate cortex in the rhesus monkey. J Physiol. 1978 Apr;277:227–244. doi: 10.1113/jphysiol.1978.sp012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. M. The projections to the superior temporal sulcus from areas 17 and 18 in the rhesus monkey. Proc R Soc Lond B Biol Sci. 1976 Apr 13;193(1111):199–207. doi: 10.1098/rspb.1976.0040. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. J Physiol. 1978 Apr;277:273–290. doi: 10.1113/jphysiol.1978.sp012272. [DOI] [PMC free article] [PubMed] [Google Scholar]