Abstract
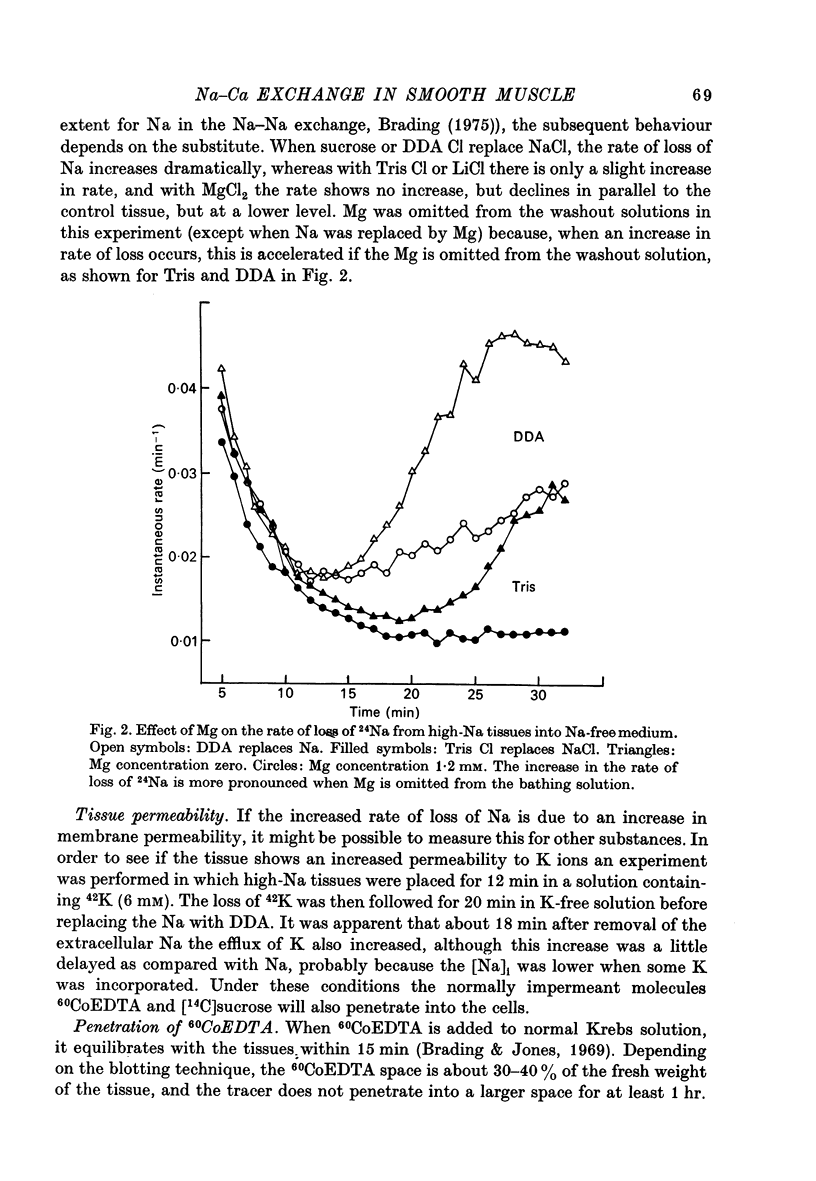
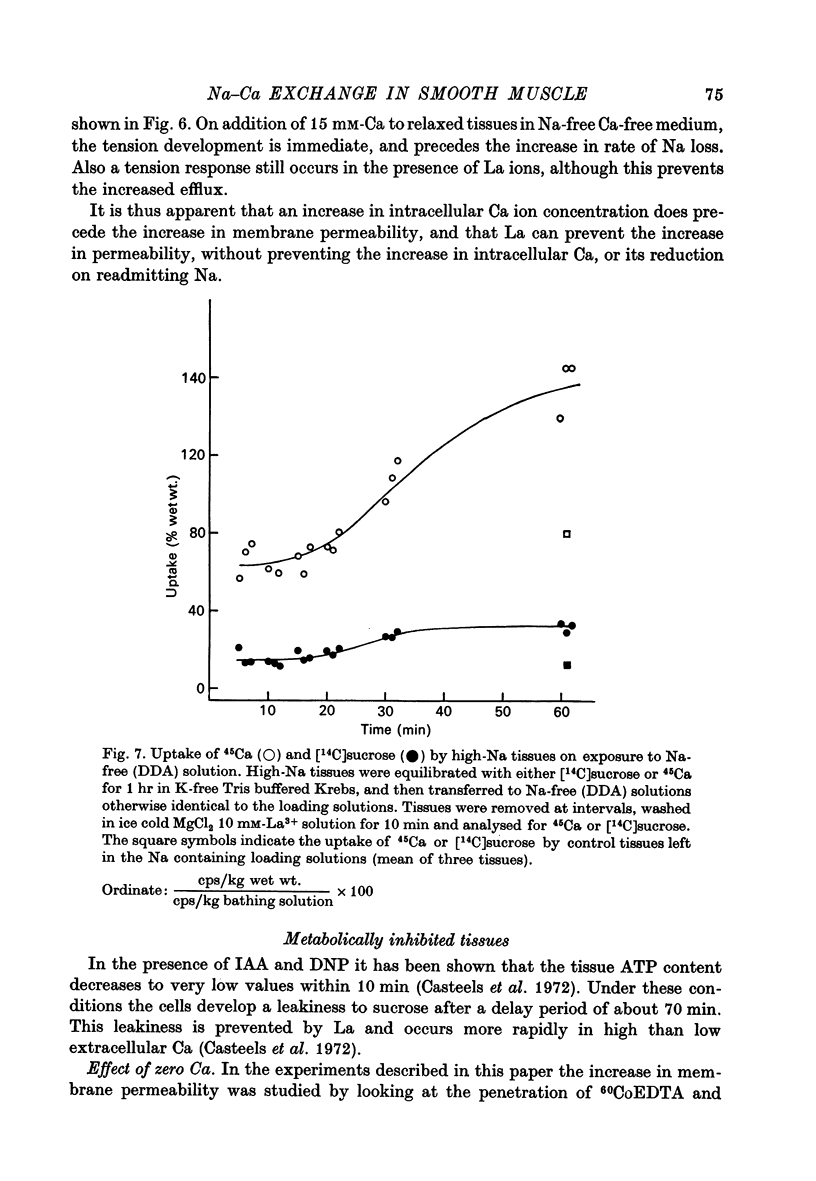
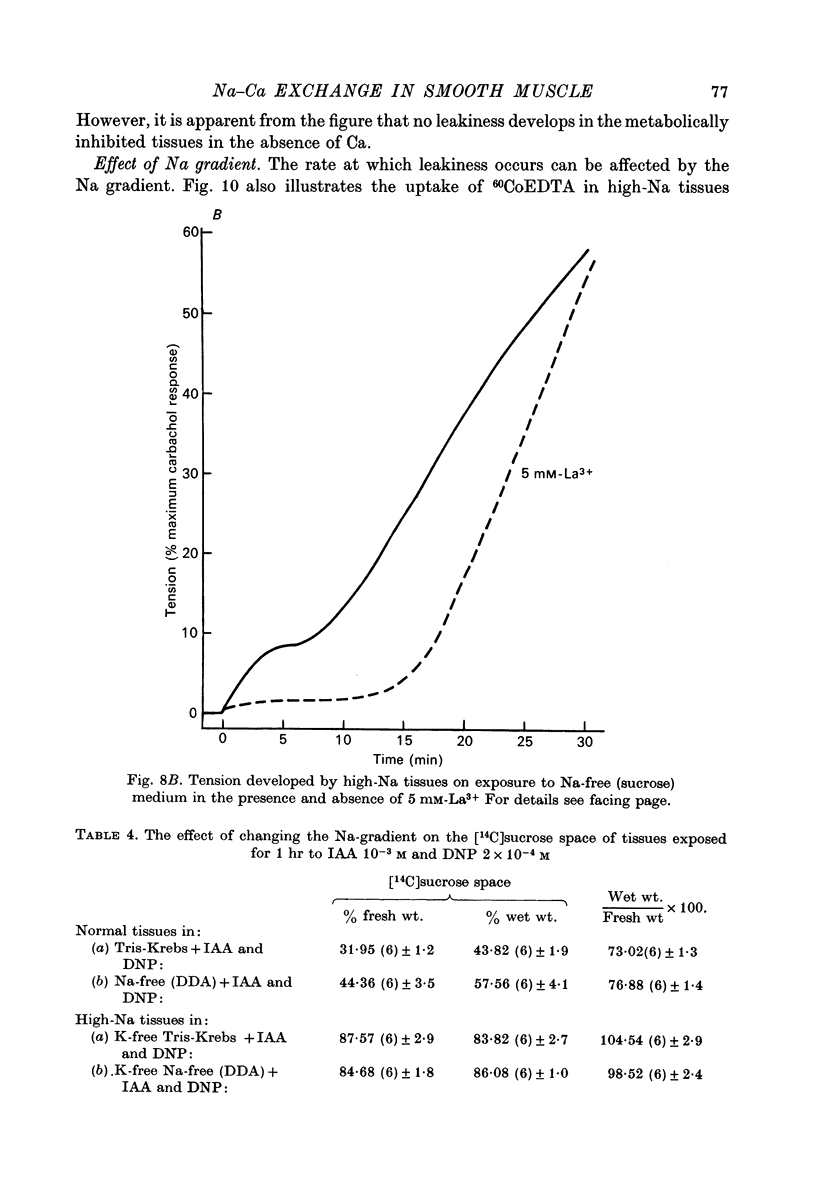
1. High-Na tissues exposed to a Na-free solution with dimethyldiethanol ammonium chloride (DDA) or sucrose replacing Na, develop an increase in membrane permeability to small ions and molecules such as Na, K, sucrose and CoEDTA. 2. The increase in permeability only occurs when the Na gradient across the cell membrane is reversed, and is not due to damaging effects of the Na-free solution. It does not occur in normal or high-K tissues, and 15 mM-[Na]0 is enough to prevent the permeability change in high-Na tissues. 3. Tissues with increased permeability maintain high levels of Ca and the increased permeability does not occur in Ca-free solutions, or in solutions containing 5 mM-La3+. The rate of development of membrane leakiness depends on the level of extracellular Ca. 4. Tissues exposed to iodoacetic acid (IAA) and dinitrophenol (DNP) also develop a membrane leakiness, dependent on extracellular Ca and blocked by La3+. 5. The time taken for development of the increase in membrane permeability in metabolically inhibited tissues can be affected by the Na gradient. With no gradient, or a slightly reversed gradient the membrane break-down occurs more rapidly. 6. It is concluded that the increase in permeability is caused by an increase in internal Ca ions, and that the Na gradient as well as the levels of ATP are important in controlling Ca movements. 7. Tension recordings also support the some form of Na--Ca exchange mechanism operating in the taenia, and this mechanism is not completely blocked by La3+ ions, although they suppress the break-down in membrane permeability.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson J., Holmberg B. The effects of K plus -free solution on tension development in the smooth muscle taenia coli from the guinea pig. Acta Physiol Scand. 1971 Jul;82(3):322–332. doi: 10.1111/j.1748-1716.1971.tb04973.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger C., Einwächter H. M., Haas H. G., Kern R. Calcium-sodium antagonism on the frog's heart: a voltage-clamp study. J Physiol. 1976 Aug;259(3):617–645. doi: 10.1113/jphysiol.1976.sp011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Analysis of the effluxes of sodium, potassium and chloride ions from smooth muscle in normal and hypertonic solutions. J Physiol. 1971 May;214(3):393–416. doi: 10.1113/jphysiol.1971.sp009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Jones A. W. Distribution and kinetics of CoEDTA in smooth muscle, and its use as an extracellular marker. J Physiol. 1969 Feb;200(2):387–401. doi: 10.1113/jphysiol.1969.sp008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Sodium/sodium exchange in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1975 Sep;251(1):79–105. doi: 10.1113/jphysiol.1975.sp011082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Widdicombe J. H. The use of lanthanum to estimate the numbers of extracellular cation-exchanging sites in the guinea-pig's taenia coli, and its effects on transmembrane monovalent ion movements. J Physiol. 1977 Apr;266(2):255–273. doi: 10.1113/jphysiol.1977.sp011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Van Breemen C. Active and passive Ca2+ fluxes across cell membranes of the guinea-pig taenia coli. Pflugers Arch. 1975 Sep 9;359(3):197–207. doi: 10.1007/BF00587379. [DOI] [PubMed] [Google Scholar]
- Casteels R., van Breemen C., Wuytack F. Effect of metabolic depletion on the membrane permeability of smooth muscle cells and its modification by La 3+ . Nat New Biol. 1972 Oct 25;239(95):249–251. doi: 10.1038/newbio239249a0. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Robinson K. Effects of inhibitors of metabolism on adenine nucleotides and on 22 Na and 42 K and net movements in rat uteri at 25 degrees C. Can J Physiol Pharmacol. 1971 Mar;49(3):205–239. doi: 10.1139/y71-026. [DOI] [PubMed] [Google Scholar]
- Imai S., Takeda K. Actions of calcium and certain multivalent cations on potassium contracture of guinea-pig's taenia coli. J Physiol. 1967 May;190(1):155–169. doi: 10.1113/jphysiol.1967.sp008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata H., Kao C. Y. Ionic currents in the guinea-pig taenia coli. J Physiol. 1976 Feb;255(2):347–378. doi: 10.1113/jphysiol.1976.sp011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katase T., Tomita T. Influences of sodium and calcium on the recovery process from potassium contracture in the guinea-pig taenia coli. J Physiol. 1972 Jul;224(2):489–500. doi: 10.1113/jphysiol.1972.sp009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. J., Moisescu D. G. The effects of very low external calcium and sodium concentrations on cardiac contractile strength and calcium-sodium antagonism. J Physiol. 1976 Jul;259(2):283–308. doi: 10.1113/jphysiol.1976.sp011466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeymaekers L., Wuytack F., Casteels R. Na-Ca exchange in Taenia coli of the guinea-pig. Pflugers Arch. 1974 Mar 25;347(4):329–340. doi: 10.1007/BF00587173. [DOI] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. Calcium-dependent potassium exchange in human red cell ghosts. J Physiol. 1976 Mar;256(1):227–244. doi: 10.1113/jphysiol.1976.sp011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. Factors controlling myogenic activity in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):73–85. doi: 10.1098/rstb.1973.0010. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Wuytack F., Casteels R., Martinelli B., Campailla E., Ferrari G. Stimulation of 45Ca efflux from smooth muscle cells by metabolic inhibition and high K depolarization. Pflugers Arch. 1975 Sep 9;359(3):183–196. doi: 10.1007/BF00587378. [DOI] [PubMed] [Google Scholar]
- Vassort G. Influence of sodium ions on the regulation of frog myocardial contractility. Pflugers Arch. 1973 Mar 30;339(3):224–240. doi: 10.1007/BF00587374. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Proceedings: The effect of lanthanum on ion content and movement in the guinea-pig's taenia coli. J Physiol. 1974 Sep;241(2):106P–107P. [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


