Abstract
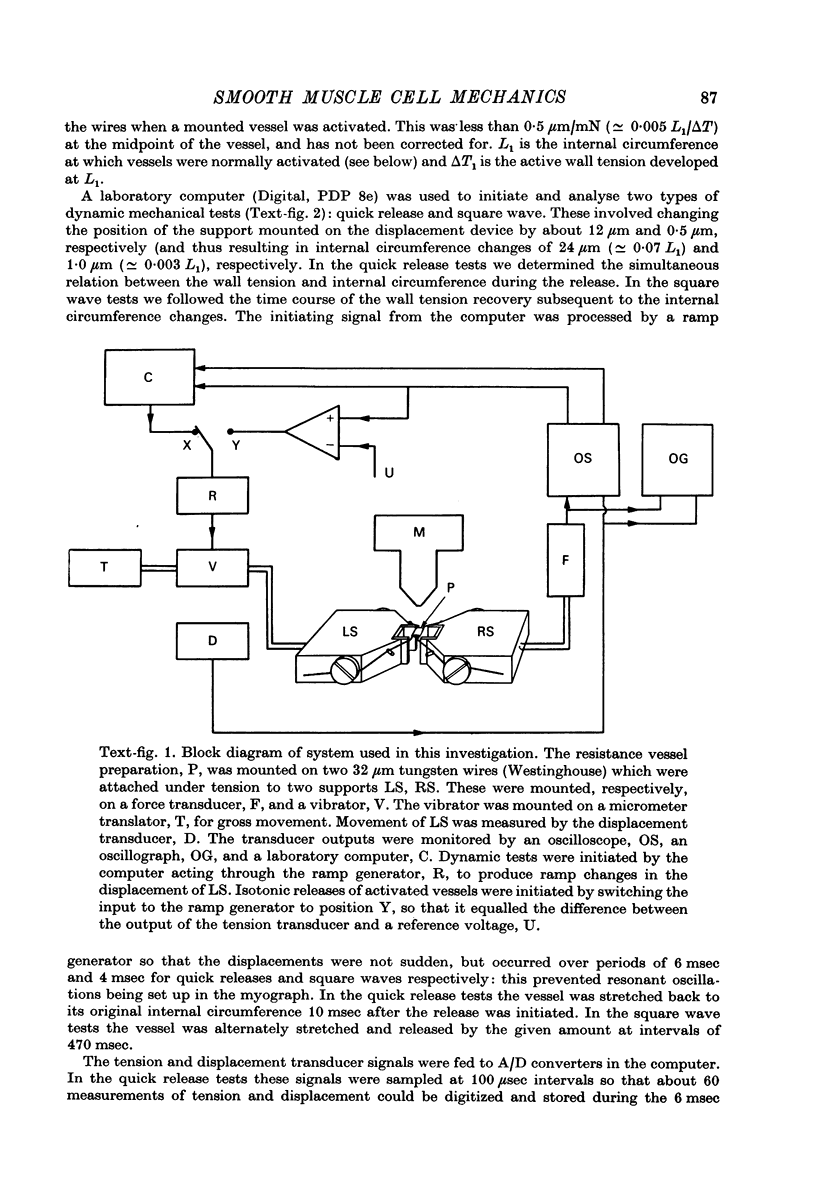
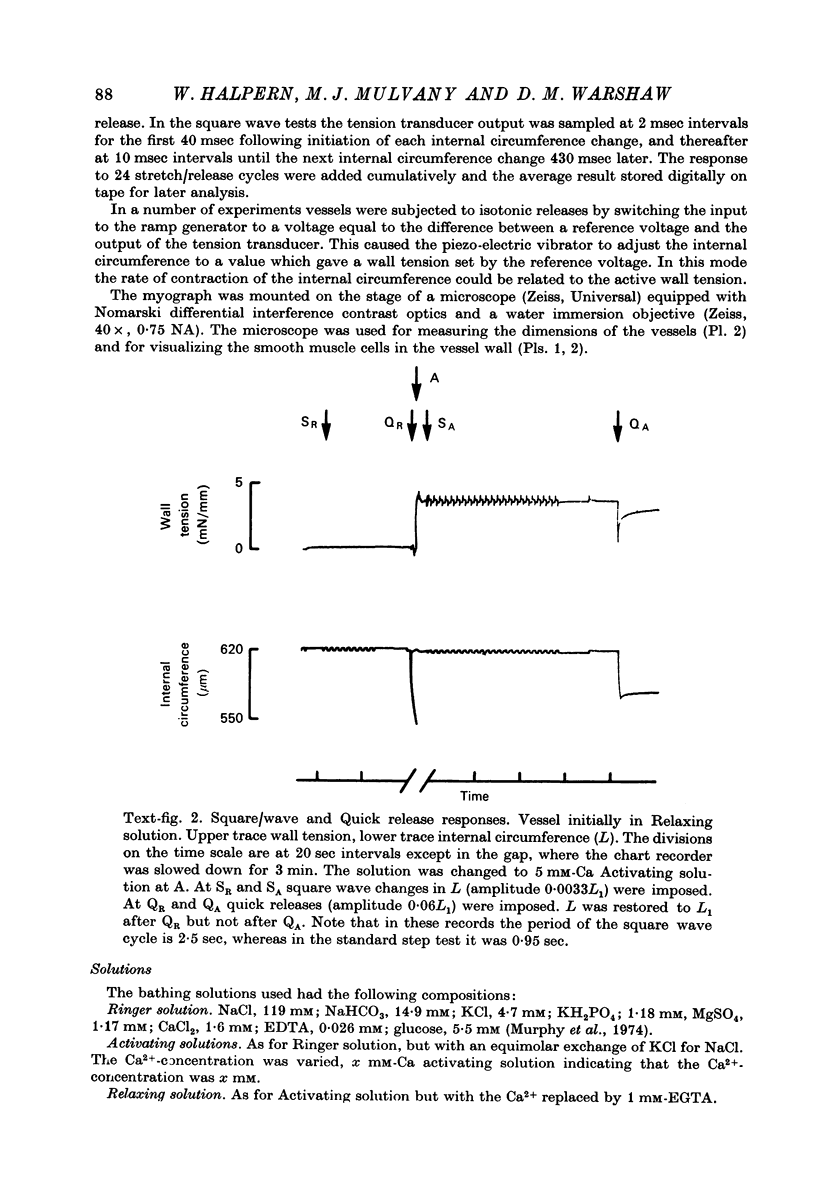
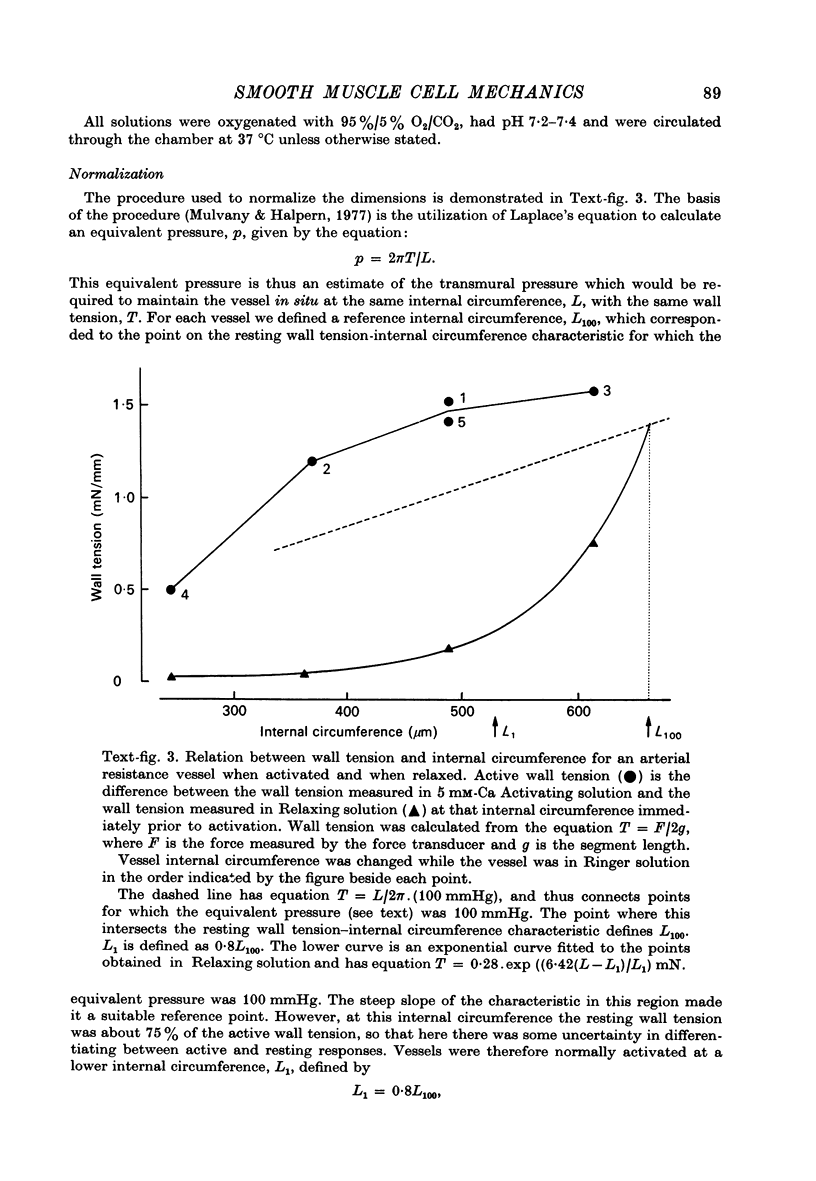
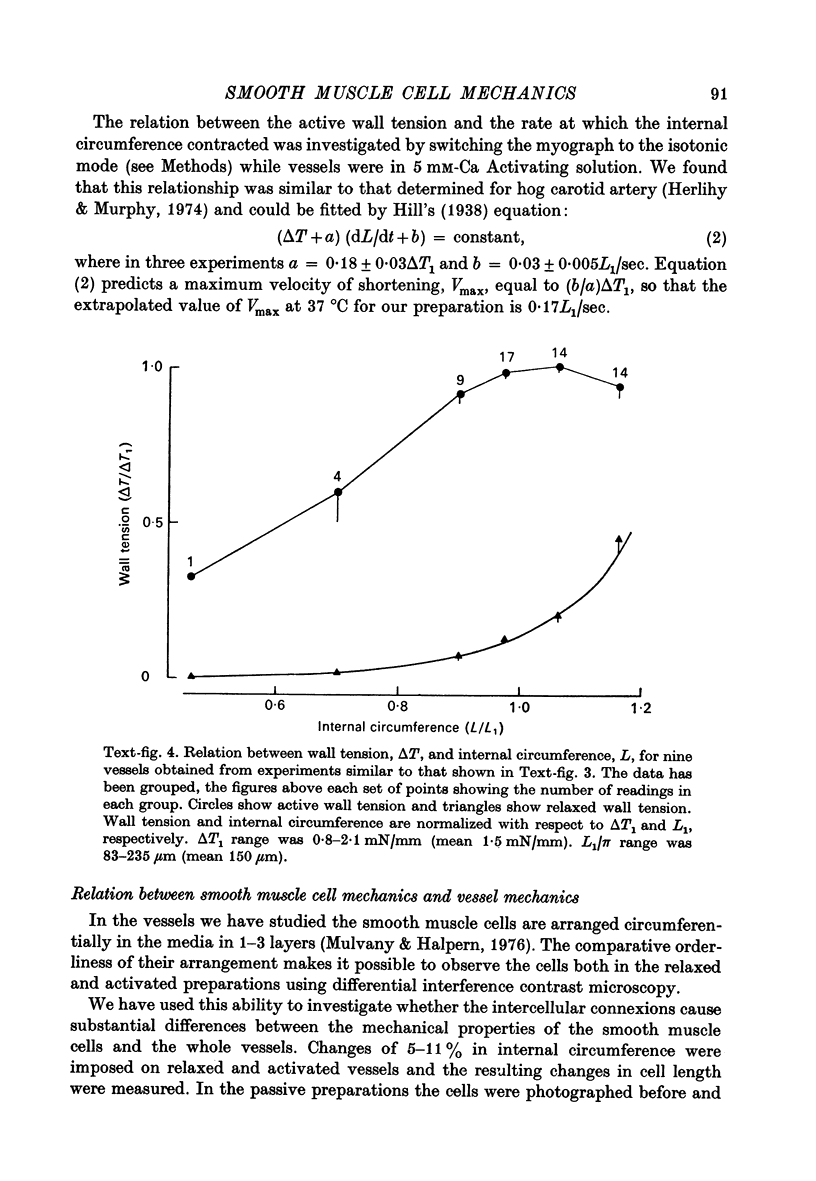
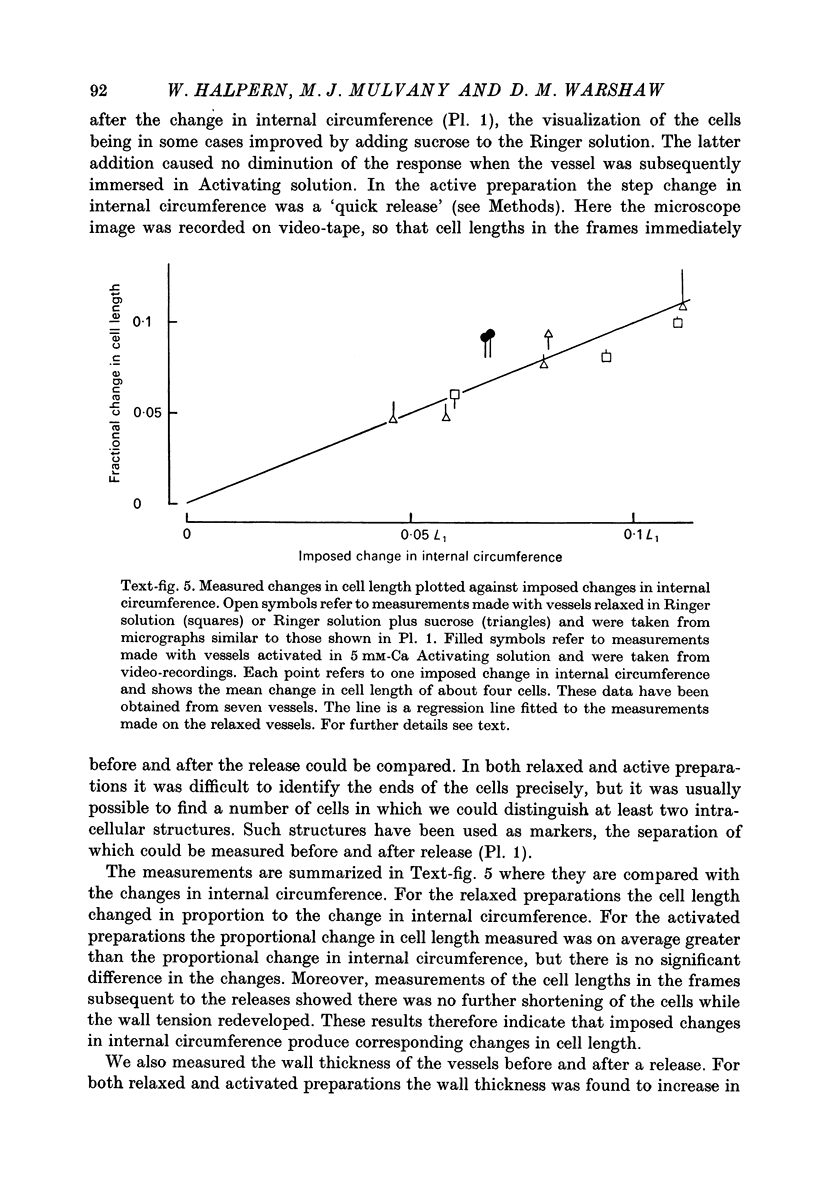
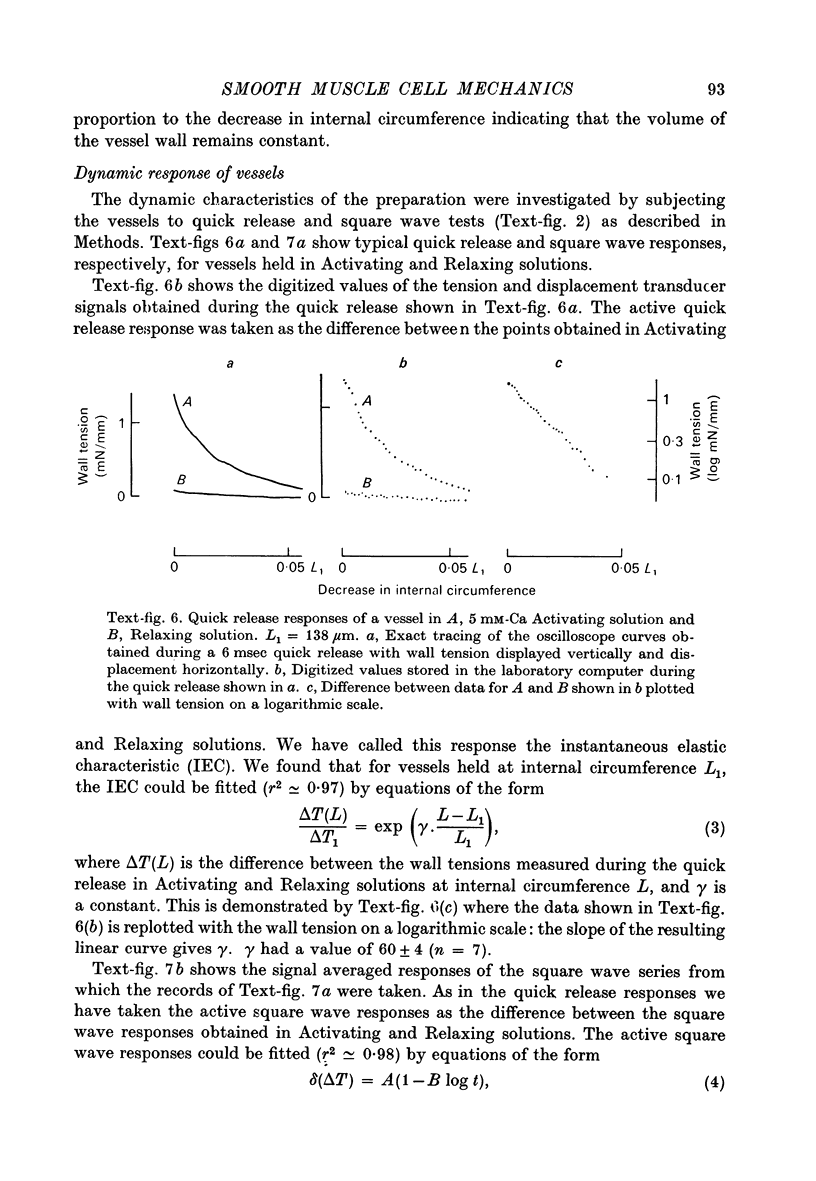
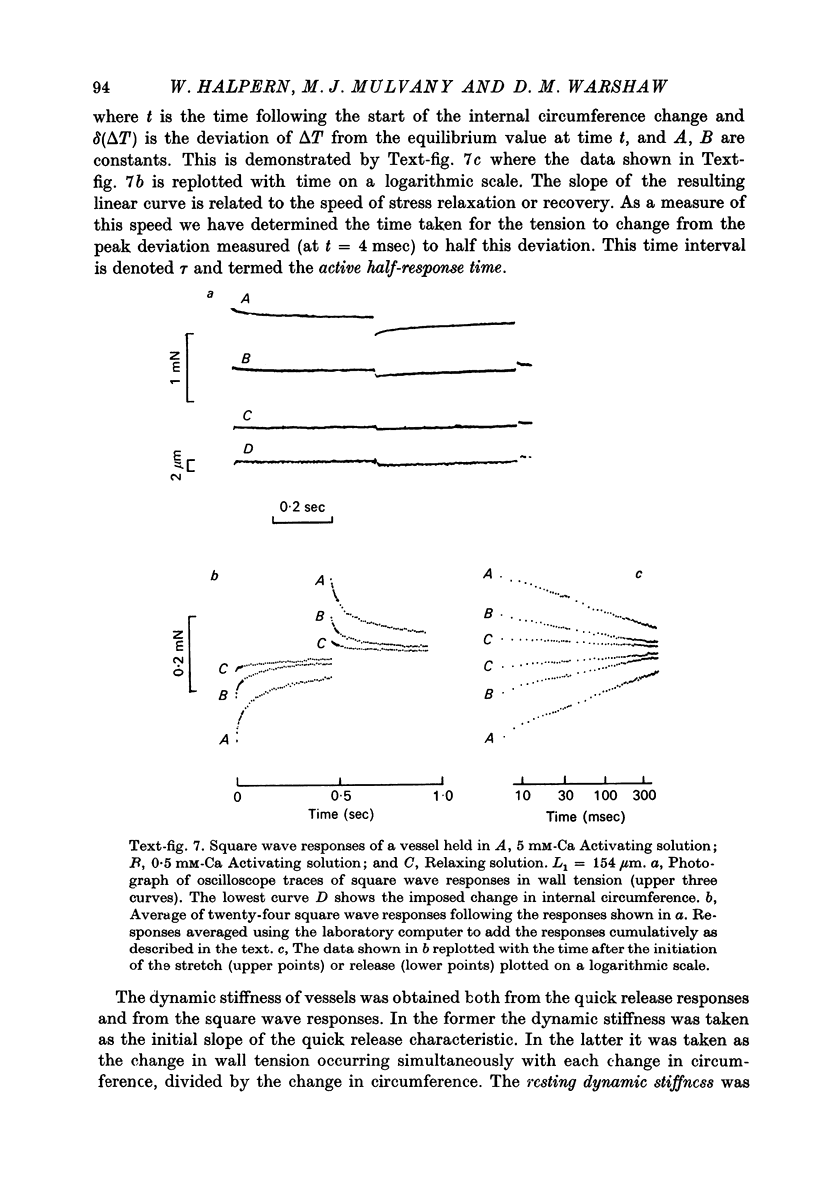
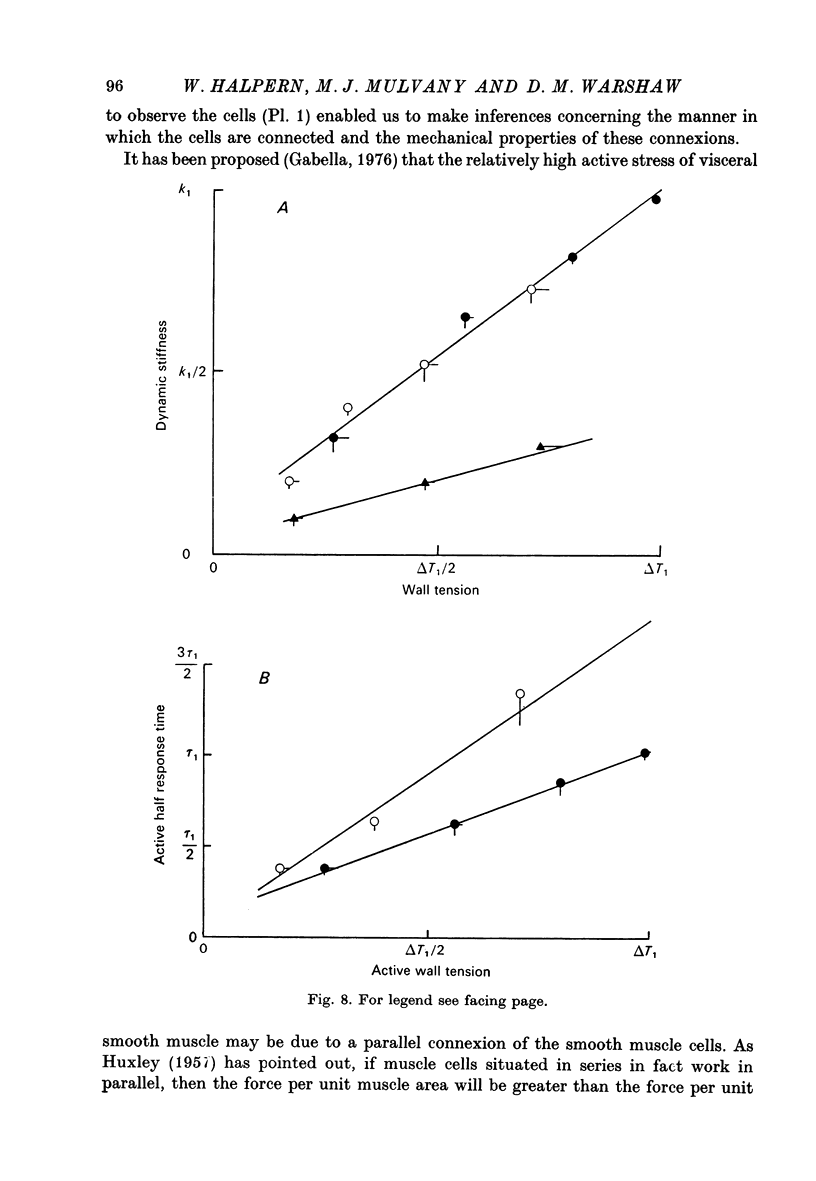
1. Methods have been developed for measuring the dynamic mechanical response of arterial resistance vessels (i.d. 83--235 micrometer) with a time resolution of about 4 msec. 2. Observations of the microscope image of the smooth muscle cells in the walls of these vessels indicate that there is little intercellular compliance in this preparation, and that the mechanical properties of the activated preparation are a reflexion of the mechanical properties of the individual smooth muscle cells. 3. Under isometric conditions the force developed per unit cell area was about 350 mN/mm2. Under isotonic conditions the cells had a maximum velocity for shortening at 37 degrees C of about 0.17 lengths/sec. 4. Quick releases of activated vessels indicate that the instantaneous elastic characteristic of smooth muscle cells is approximately exponential. 5. The wall tension response to small (0.3%) square wave changes in circumference was proportional to the logarithm of the time following the start of each circumference change. 6. Active wall tension, deltaT, was varied by varying the Ca2+ concentration of the activating solution. Under these conditions the active dynamic stiffness, k, was proportional to deltaT, and was not temperature dependent. The active half response time, tau (the time, taken to recover half the tension change caused by a small change in circumference) was also proportional to deltaT, but here the constant of proportionality had a Q10 of about 1.8. 7. It is concluded that the quick release response and the square wave response are in part a function of the mechanical properties of the crossbridges between the contractile filaments. Calculations show that both these responses can be explained if it is assumed that there is a relatively compliant passive component in series with the crossbridges.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. S. Series elasticity of urinary bladder smooth muscle. Am J Physiol. 1976 Nov;231(5 Pt 1):1337–1342. doi: 10.1152/ajplegacy.1976.231.5.1337. [DOI] [PubMed] [Google Scholar]
- Ashton F. T., Somlyo A. V., Somlyo A. P. The contractile apparatus of vascular smooth muscle: intermediate high voltage stereo electron microscopy. J Mol Biol. 1975 Oct 15;98(1):17–29. doi: 10.1016/s0022-2836(75)80098-2. [DOI] [PubMed] [Google Scholar]
- Bagby R. M., Fisher B. A. Graded contractions in muscle strips and single cells from Bufo marinus stomach. Am J Physiol. 1973 Jul;225(1):105–109. doi: 10.1152/ajplegacy.1973.225.1.105. [DOI] [PubMed] [Google Scholar]
- Bagby R. M., Young A. M., Dotson R. S., Fisher B. A., McKinnon K. Contraction of single smooth muscle cells from Bufo marinus stomach. Nature. 1971 Dec 10;234(5328):351–352. doi: 10.1038/234351a0. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Chamley J. H. Thick filaments in vertebrate smooth muscle. Cell Tissue Res. 1975;156(2):201–216. doi: 10.1007/BF00221803. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Fay F. S. Correlation between fiber length, ultrastructure, and the length-tension relationship of mammalian smooth muscle. J Cell Biol. 1972 Jan;52(1):105–116. doi: 10.1083/jcb.52.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Fisher B. A., Bagby R. M. Reorientation of myofilaments during contraction of a vertebrate smooth muscle. Am J Physiol. 1977 Jan;232(1):C5–14. doi: 10.1152/ajpcell.1977.232.1.C5. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The instantaneous elasticity of frog skeletal muscle fibres [proceedings]. J Physiol. 1976 Sep;260(2):28P–29P. [PubMed] [Google Scholar]
- Gabella G. The force generated by a visceral smooth muscle. J Physiol. 1976 Dec;263(2):199–213. doi: 10.1113/jphysiol.1976.sp011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven K., Rudolph K. H., Hohorst B. Creep after loading in the relaxed and contracted smooth muscle (taenia coli of the guinea pig) under various osmotic conditions. Pflugers Arch. 1976 Apr 6;362(3):255–260. doi: 10.1007/BF00581178. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Herlihy J. T., Murphy R. A. Force-velocity and series elastic characteristics of smooth muscle from the hog carotid artery. Circ Res. 1974 Apr;34(4):461–466. doi: 10.1161/01.res.34.4.461. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R. Variation of muscle stiffness with force at increasing speeds of shortening. J Gen Physiol. 1975 Sep;66(3):287–302. doi: 10.1085/jgp.66.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. W., Pollack G. H. Myocardial sarcomere dynamics during isometric contraction. J Physiol. 1975 Oct;251(3):627–643. doi: 10.1113/jphysiol.1975.sp011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R., Vibert P. J. Myosin filaments in vertebrate smooth muscle. Nature. 1970 Mar 14;225(5237):1053–1054. doi: 10.1038/2251053a0. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Mechanical properties of vascular smooth muscle cells in situ. Nature. 1976 Apr 15;260(5552):617–619. doi: 10.1038/260617a0. [DOI] [PubMed] [Google Scholar]
- Murphy R. A., Herlihy J. T., Megerman J. Force-generating capacity and contractile protein content of arterial smooth muscle. J Gen Physiol. 1974 Dec;64(6):691–705. doi: 10.1085/jgp.64.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROSSER C. L., BURNSTOCK G., KAHN J. Conduction in smooth muscle: comparative structural properties. Am J Physiol. 1960 Sep;199:545–552. doi: 10.1152/ajplegacy.1960.199.3.545. [DOI] [PubMed] [Google Scholar]
- ROSENBLUTH J. SMOOTH MUSCLE: AN ULTRASTRUCTURAL BASIS FOR THE DYNAMIC OF ITS CONTRACTION. Science. 1965 Jun 4;148(3675):1337–1339. doi: 10.1126/science.148.3675.1337. [DOI] [PubMed] [Google Scholar]
- Shoenberg C. F. A study of myosin filaments in extracts and homogenates of vertebrate smooth muscle. Angiologica. 1969;6(2):233–246. doi: 10.1159/000157791. [DOI] [PubMed] [Google Scholar]
- Shoenberg C. F., Needham D. M. A study of the mechanism of contraction in vertebrate smooth muscle. Biol Rev Camb Philos Soc. 1976 Feb;51(1):53–104. doi: 10.1111/j.1469-185x.1976.tb01120.x. [DOI] [PubMed] [Google Scholar]
- Small J. V. Studies on isolated smooth muscle cells: The contractile apparatus. J Cell Sci. 1977 Apr;24:327–349. doi: 10.1242/jcs.24.1.327. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., Rice R. V. Filament organization in vertebrate smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):223–229. doi: 10.1098/rstb.1973.0027. [DOI] [PubMed] [Google Scholar]