Abstract
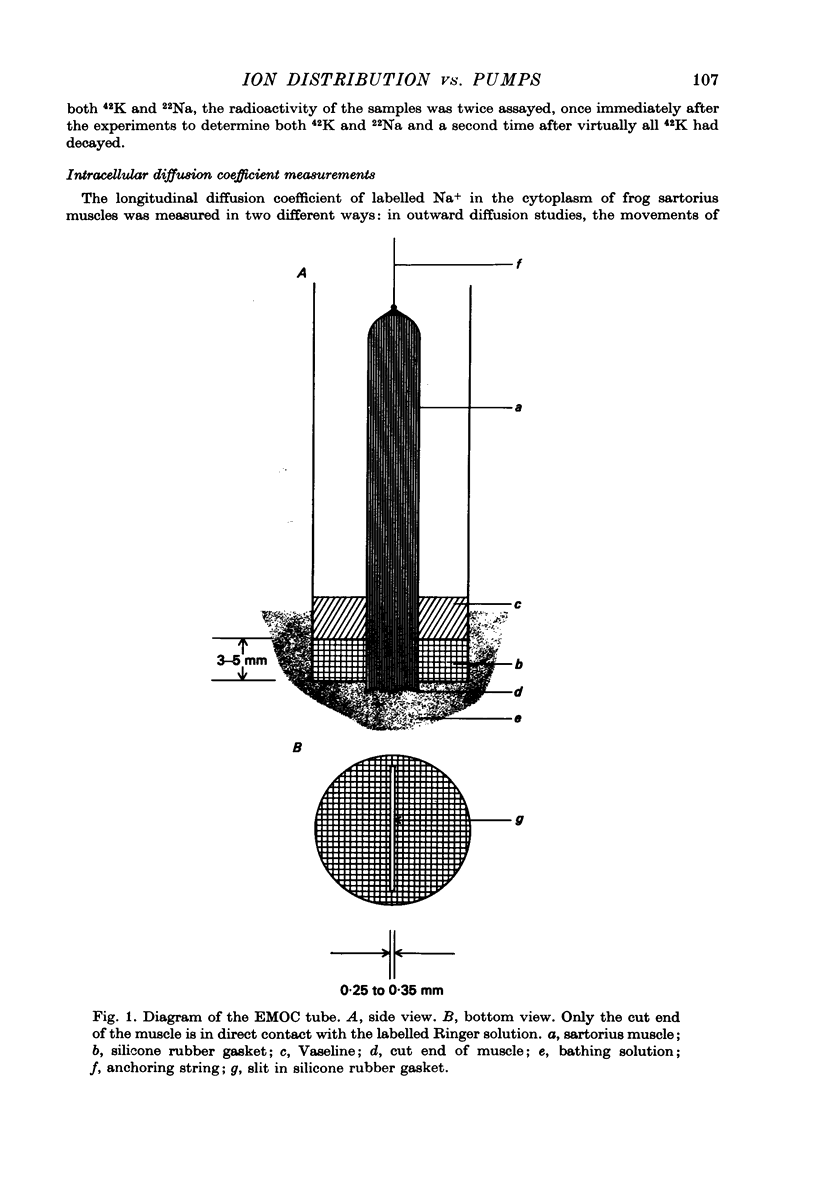
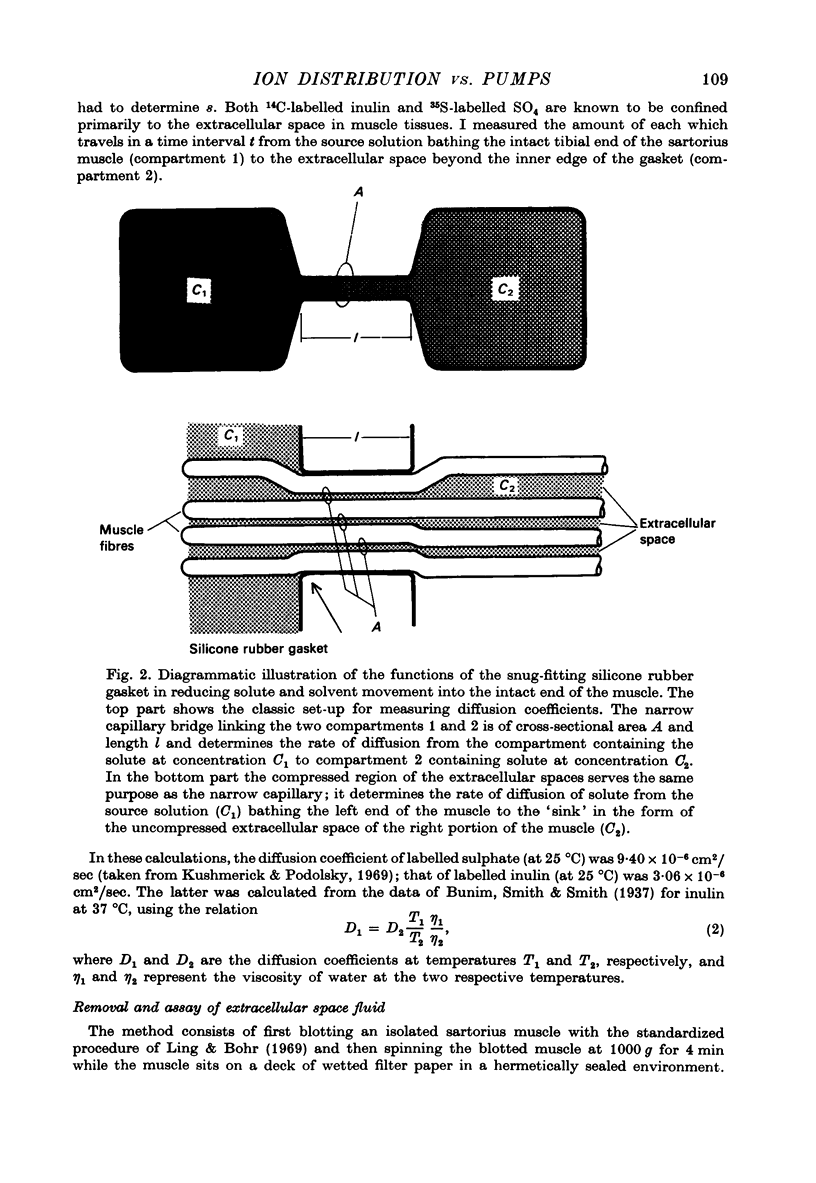
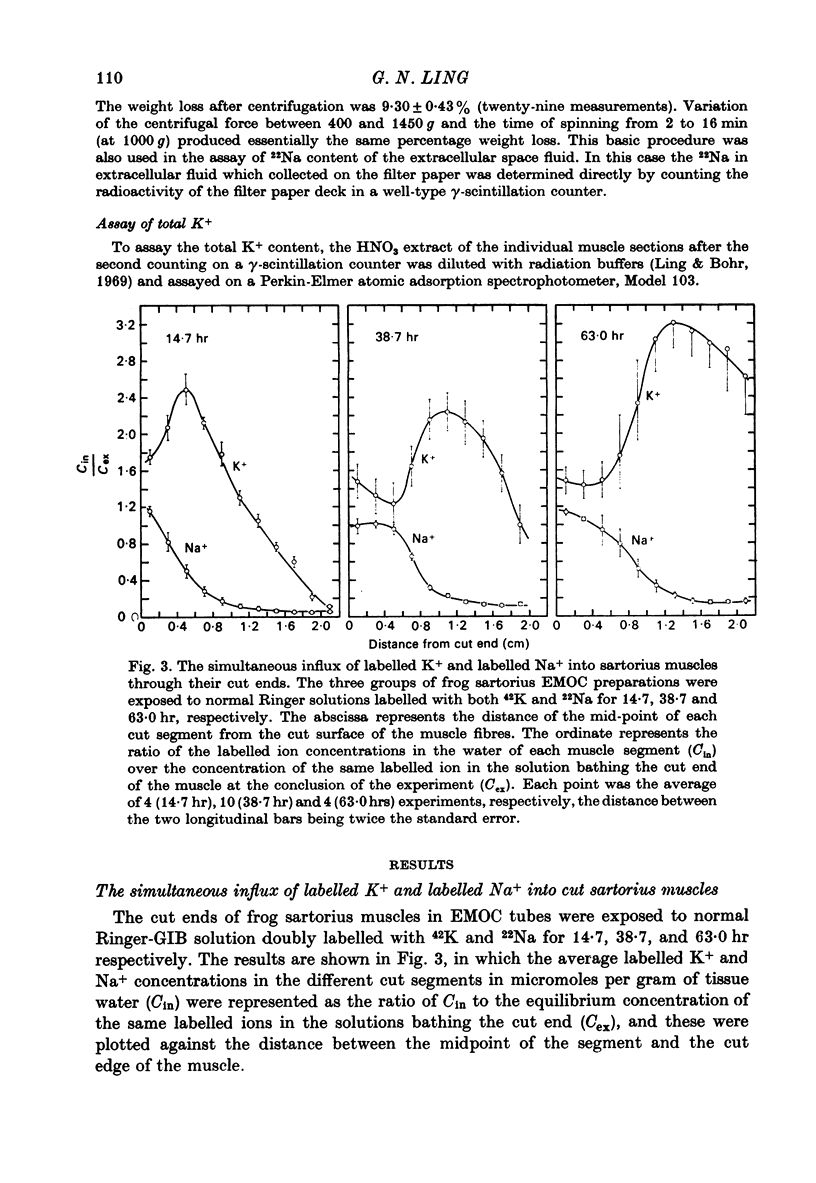
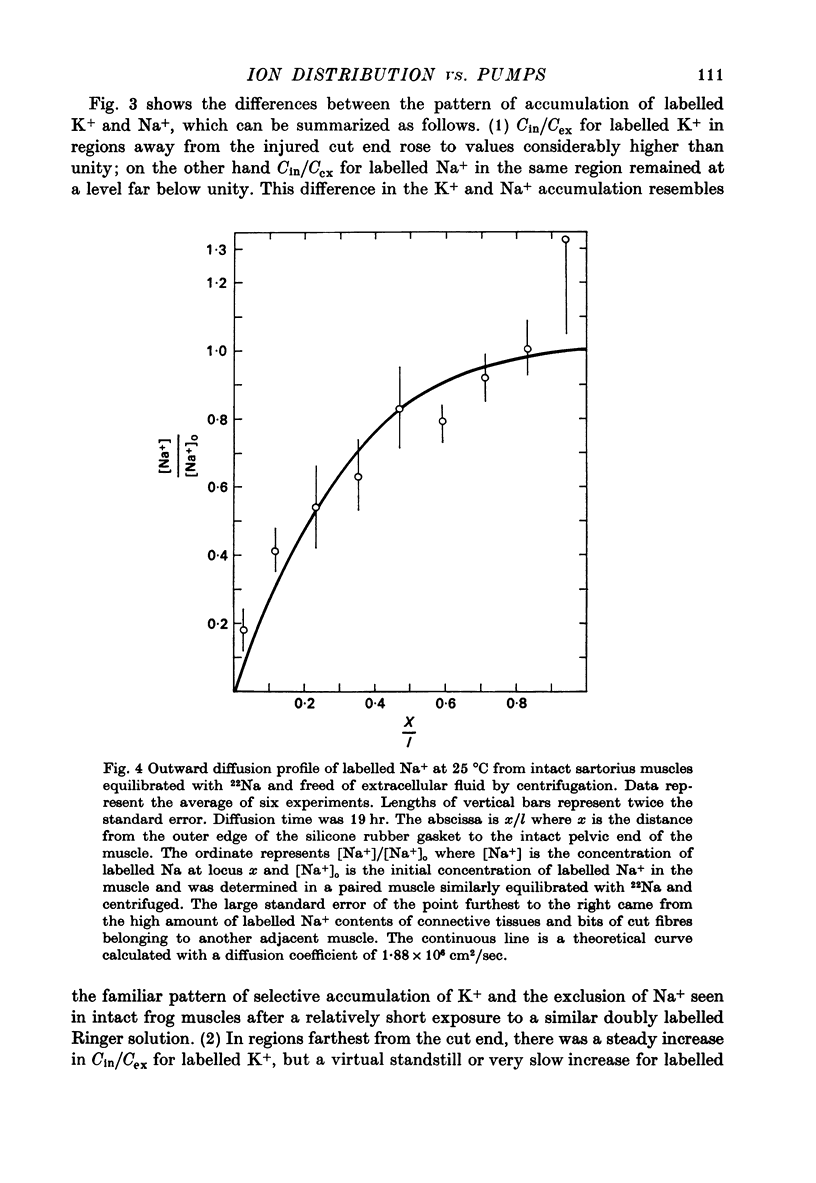
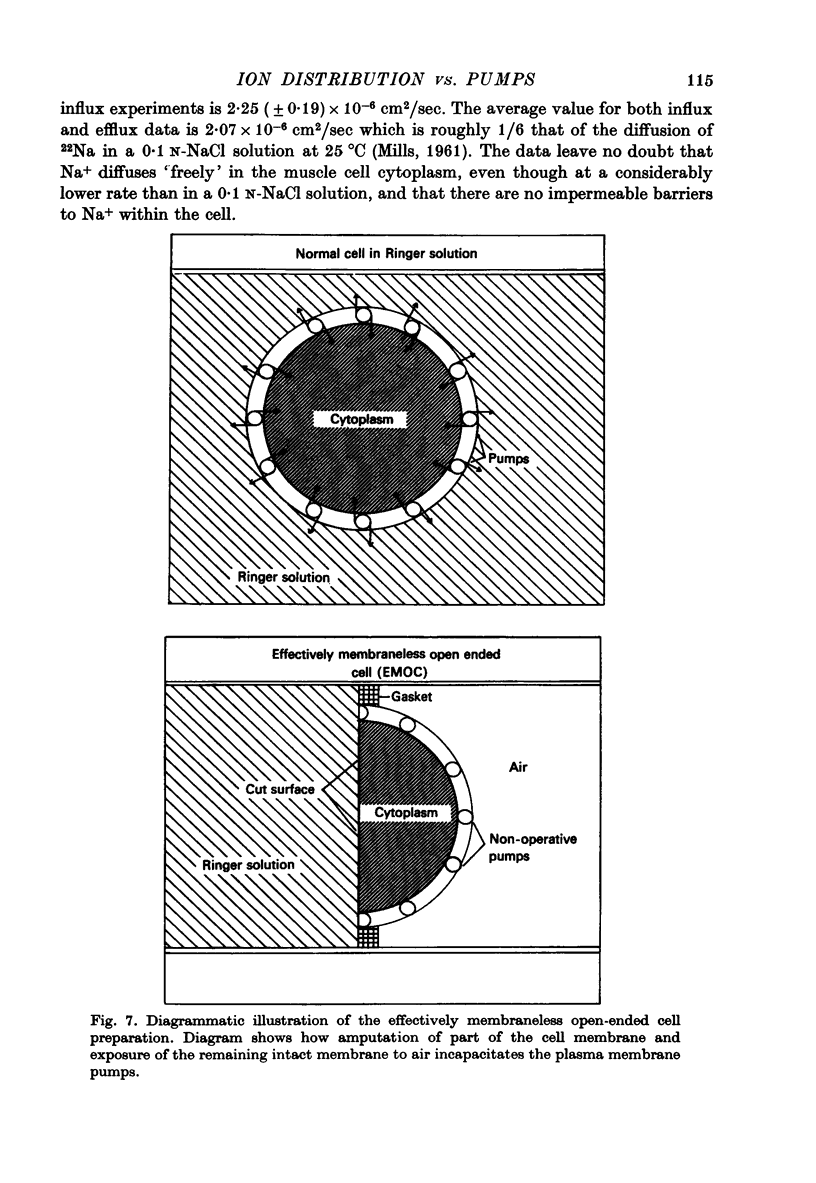
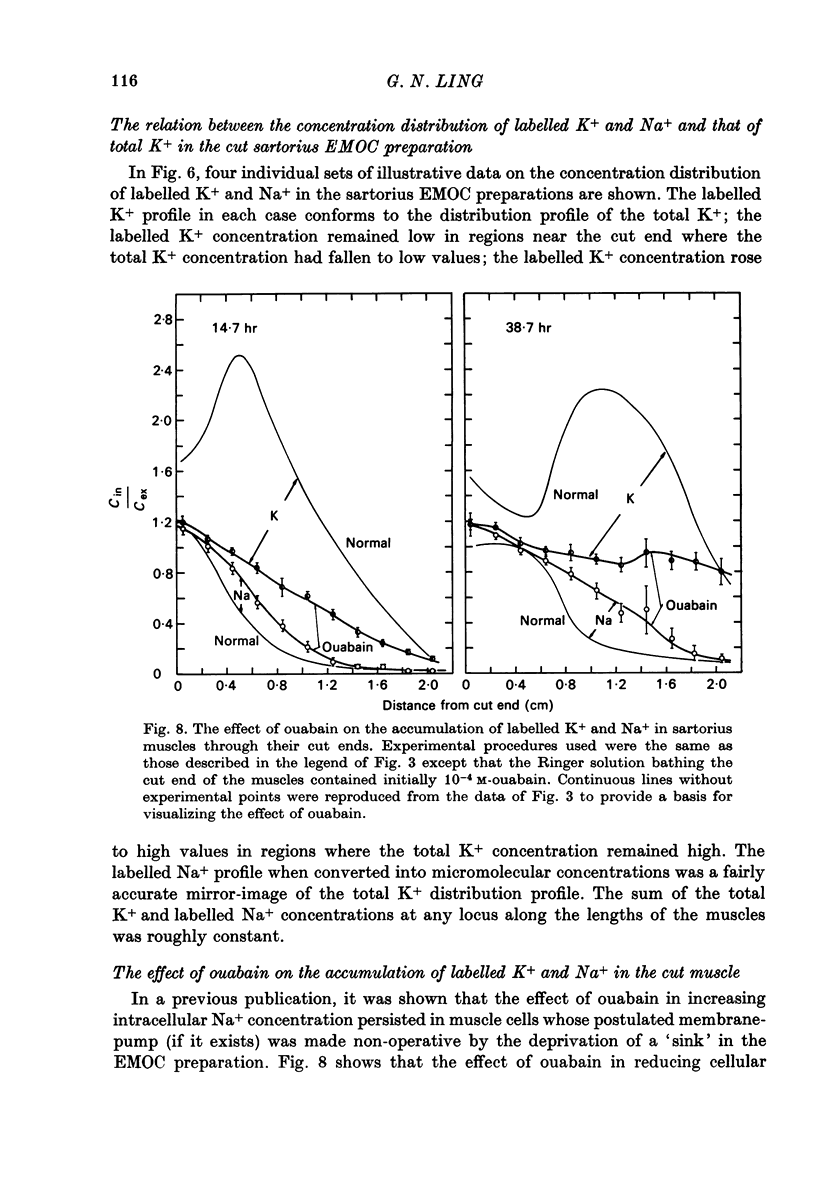
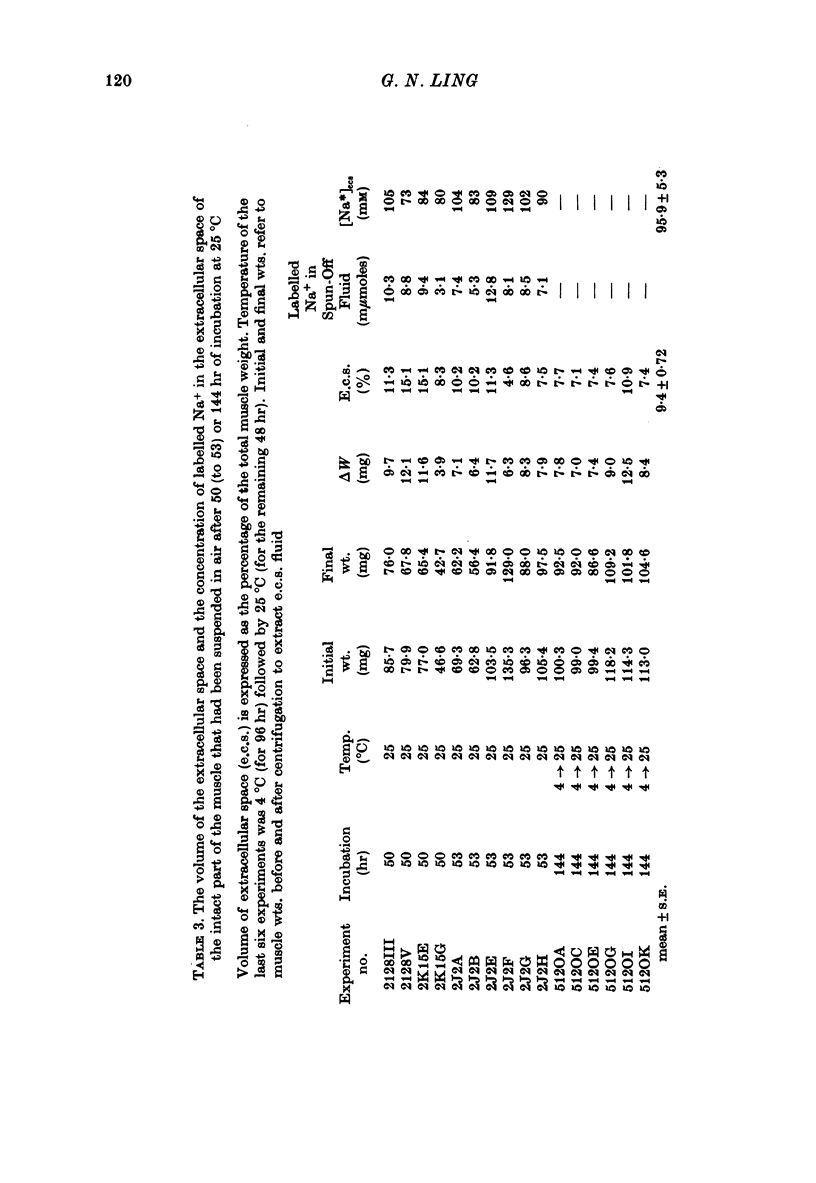
1. Previous work has shown that a frog sartorius muscle consists of parallel cells running all the way from one end of the muscle to the other and that amputation of one end of the muscle is not followed by regeneration of a new cell membrane. If now only the cut end of the amputated muscle is exposed to a Ringer solution in which the solutes 42K and 22Na act as radioactive labels and the rest of the cell is suspended in air, we have what is described as an effectively membraneless open-ended cell or EMOC preparation. In this case the only remaining anatomically intact plasma membrane and pumps are made nonfunctional by the removal of 'sources' for inward pumps and 'sinks' for outward pumps. 2. The healthy region of a frog sartorius muscle EMOC preparation continues to accumulate labelled K+ to a level higher than that in the Ringer solution and to exclude labelled Na+ to a level below that in the Ringer solution, much as a normal uncut muscle does in its normal environment. The differences were reduced by inclusion of ouabain in the medium. 3. The diffusion coefficient of Na+ in the normal muscle cytoplasm at 25 degrees C was measured using two methods. The average diffusion coefficient measured was 2.07 X 10(-6) cm2/sec, roughly 1/6 that of the diffusion coefficient of Na+ in a 0.1 N-NaCl solution. 4. The data obtained are discussed in terms of the association-induction hypothesis. In this theory asymmetrical solute distribution, basically an expression of a non-energy consuming metastable equilibrium state, is the result of specific combinations of two opposing mechanisms: adsorption which raises the level of the intracellular solute; and exclusion from cell water which tends to lower it.
Full text
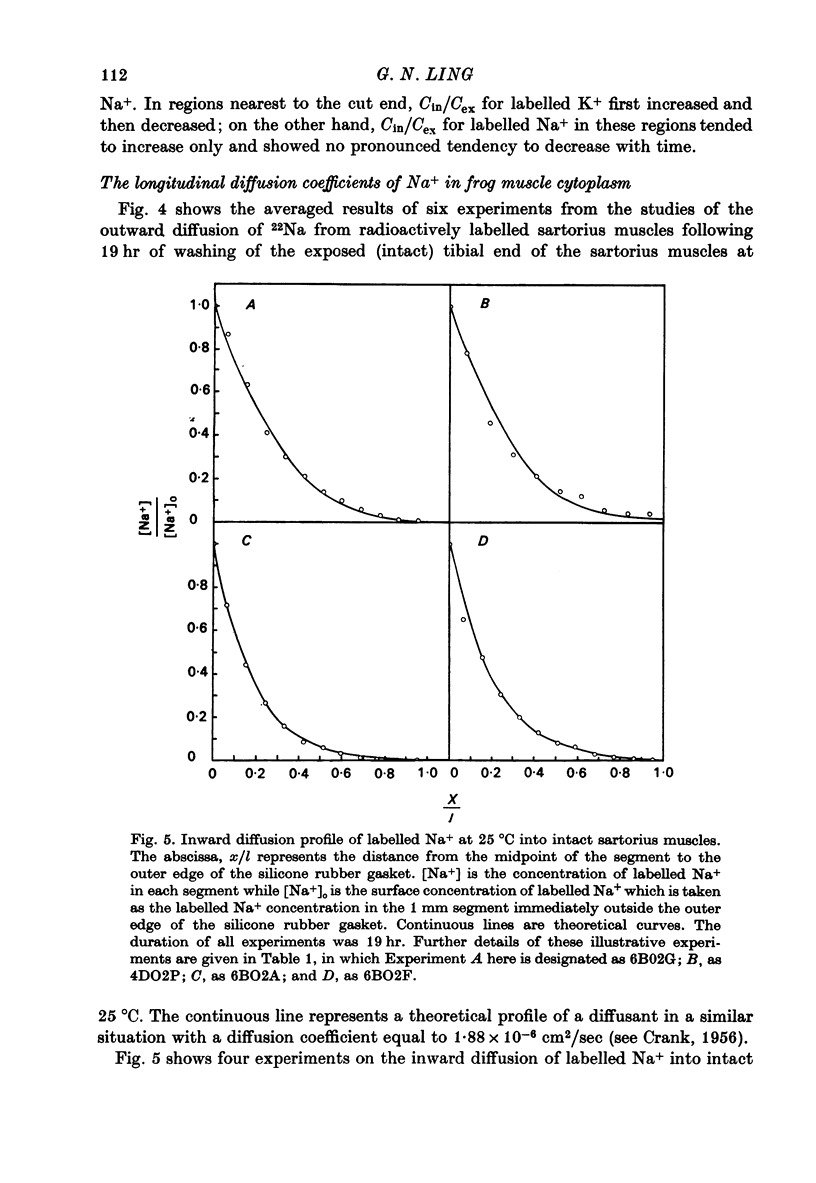
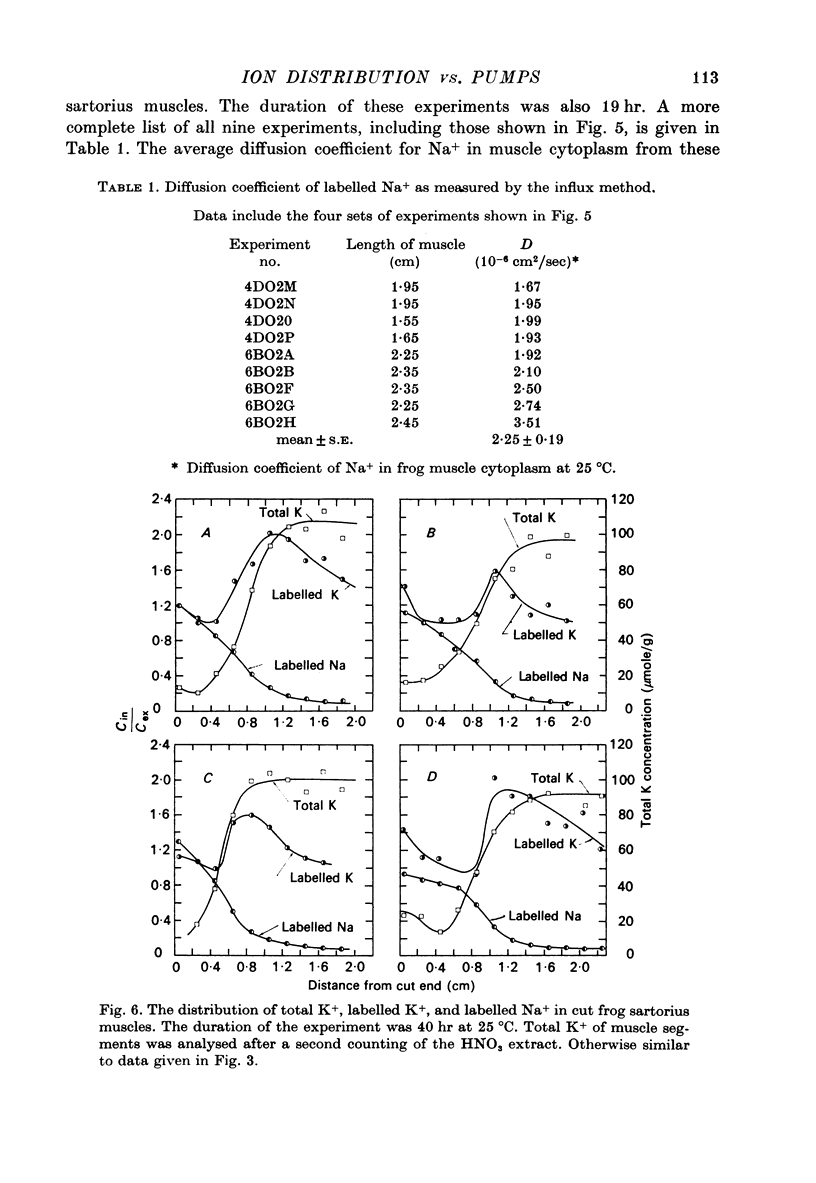
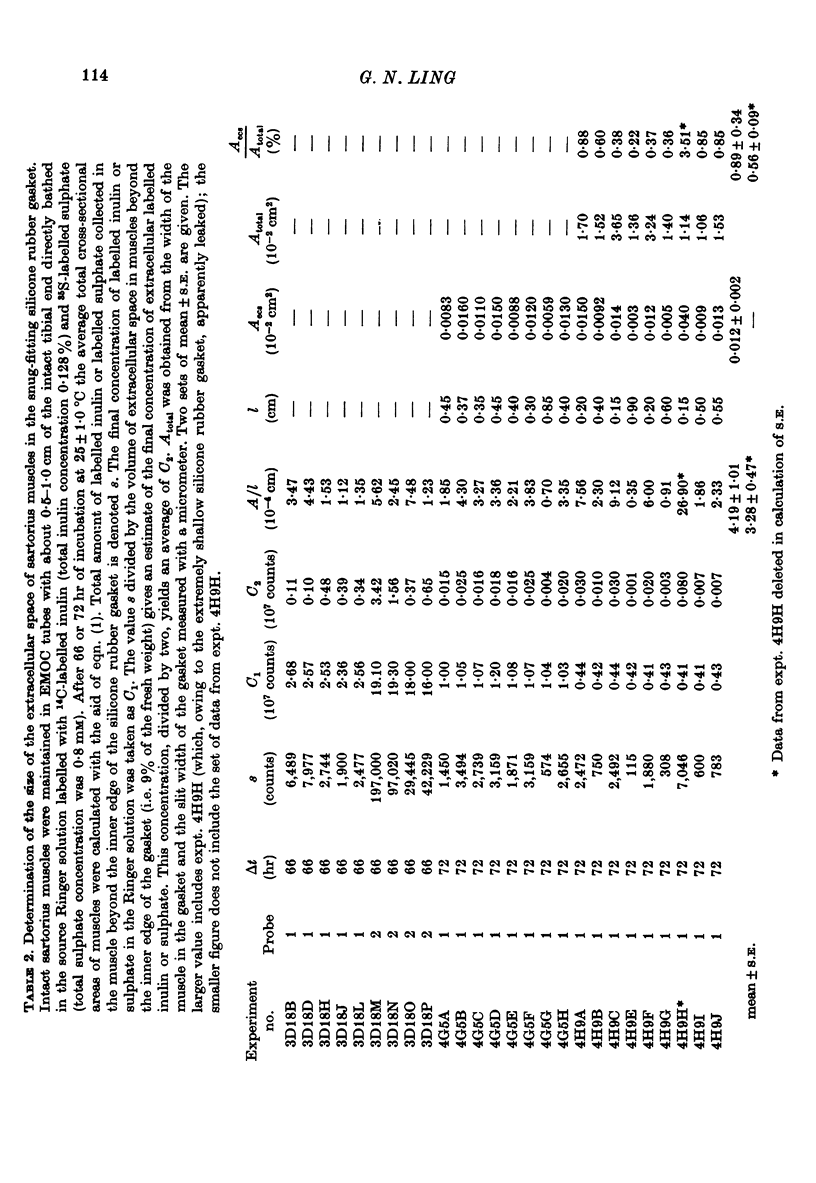
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Ochesenfeld M. M., Ling G. N. Metabolic cooperative control of electrolyte levels by adenosine triphosphate in the frog muscle. Biophys J. 1971 Dec;11(12):973–980. doi: 10.1016/s0006-3495(71)86271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- LING G. N. THE ASSOCIATION-INDUCTION HYPOTHESIS. Tex Rep Biol Med. 1964;22:244–265. [PubMed] [Google Scholar]
- Ling G. N. A new model for the living cell: a summary of the theory and recent experimental evidence in its support. Int Rev Cytol. 1969;26:1–61. doi: 10.1016/s0074-7696(08)61633-2. [DOI] [PubMed] [Google Scholar]
- Ling G. N. An answer to a reported apparent contradiction in the predicted relation between the concentration of ATP and K in living cells. Physiol Chem Phys. 1974;6(3):285–286. [PubMed] [Google Scholar]
- Ling G. N. How does ouabain control the levels of cell K+ and Na+? by interference with a Na pump or by allosteric control of K+-Na+ adsorption on cytoplasmic protein sites? Physiol Chem Phys. 1973;5(4):295–211. [PubMed] [Google Scholar]
- Ling G. N., Kromash M. H. The extracellular space of voluntary muscle tissues. J Gen Physiol. 1967 Jan;50(3):677–694. doi: 10.1085/jgp.50.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N., Miller C., Ochsenfeld M. M. The physical state of solutes and water in living cells according to the association-induction hypothesis. Ann N Y Acad Sci. 1973 Mar 30;204:6–50. doi: 10.1111/j.1749-6632.1973.tb30770.x. [DOI] [PubMed] [Google Scholar]
- Ling G. N., Ochsenfeld M. M. Control of cooperative adsorption of solutes and water in living cells by hormones, drugs, and metabolic products. Ann N Y Acad Sci. 1973 Mar 30;204:325–336. doi: 10.1111/j.1749-6632.1973.tb30788.x. [DOI] [PubMed] [Google Scholar]
- Ling G. N. Studies on ion permeability: III. Diffusion of Br-minus ion in the extracellular space of frog muscles. Physiol Chem Phys. 1972;4(3):199–208. [PubMed] [Google Scholar]
- Ling G. N., Walton C. L. A simple rapid method for the quantitative separation of the extracellular fluid in frog muslces. Physiol Chem Phys. 1975;7(3):215–218. [PubMed] [Google Scholar]
- Ling G. N., Walton C. L. What retains water in living cells? Science. 1976 Jan 23;191(4224):293–295. doi: 10.1126/science.1082166. [DOI] [PubMed] [Google Scholar]
- Minkoff L., Damadian R. Caloric catastrophe. Biophys J. 1973 Feb;13(2):167–178. doi: 10.1016/S0006-3495(73)85977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkoff L., Damadian R. Reply to letters on "caloric catastrophe": Inadequacy of the energy available from ATP for membrane transport. Biophys J. 1974 Jan;14(1):69–72. doi: 10.1016/S0006-3495(74)85903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


