Abstract
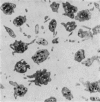
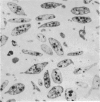
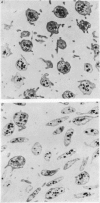
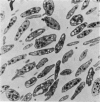
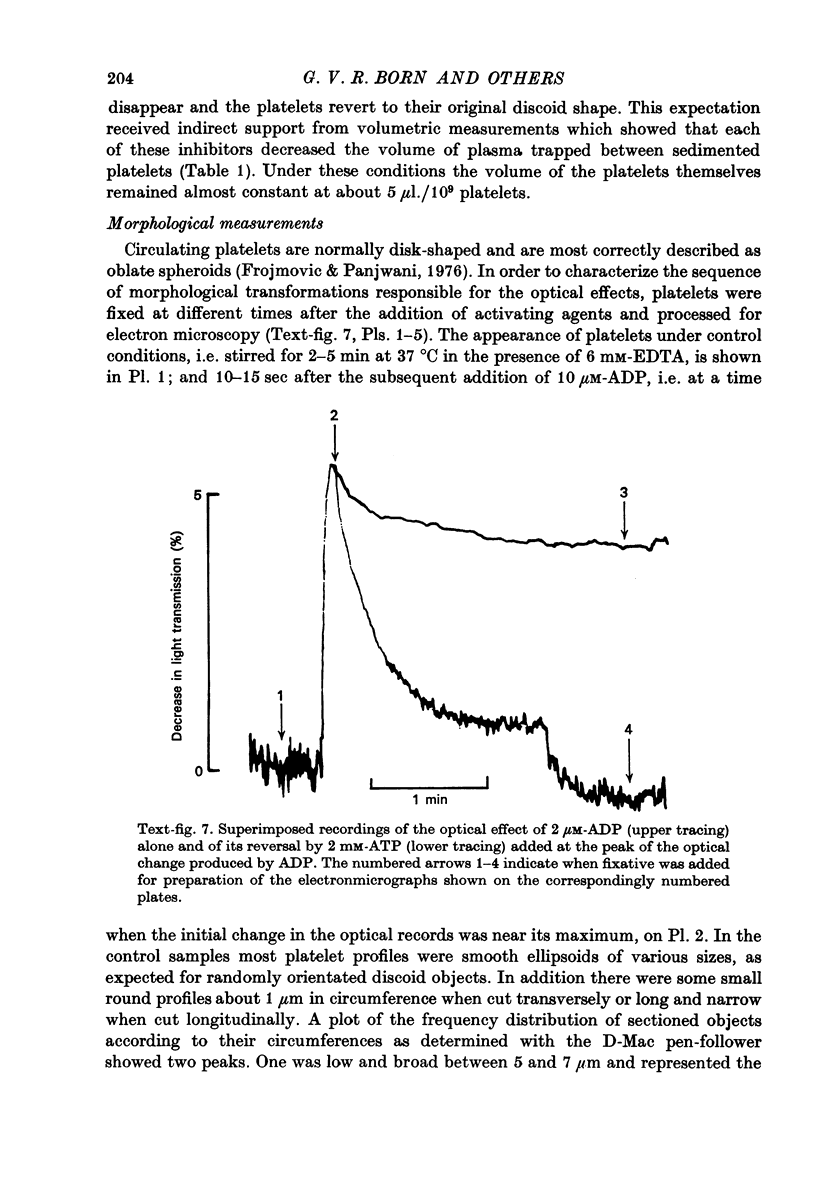
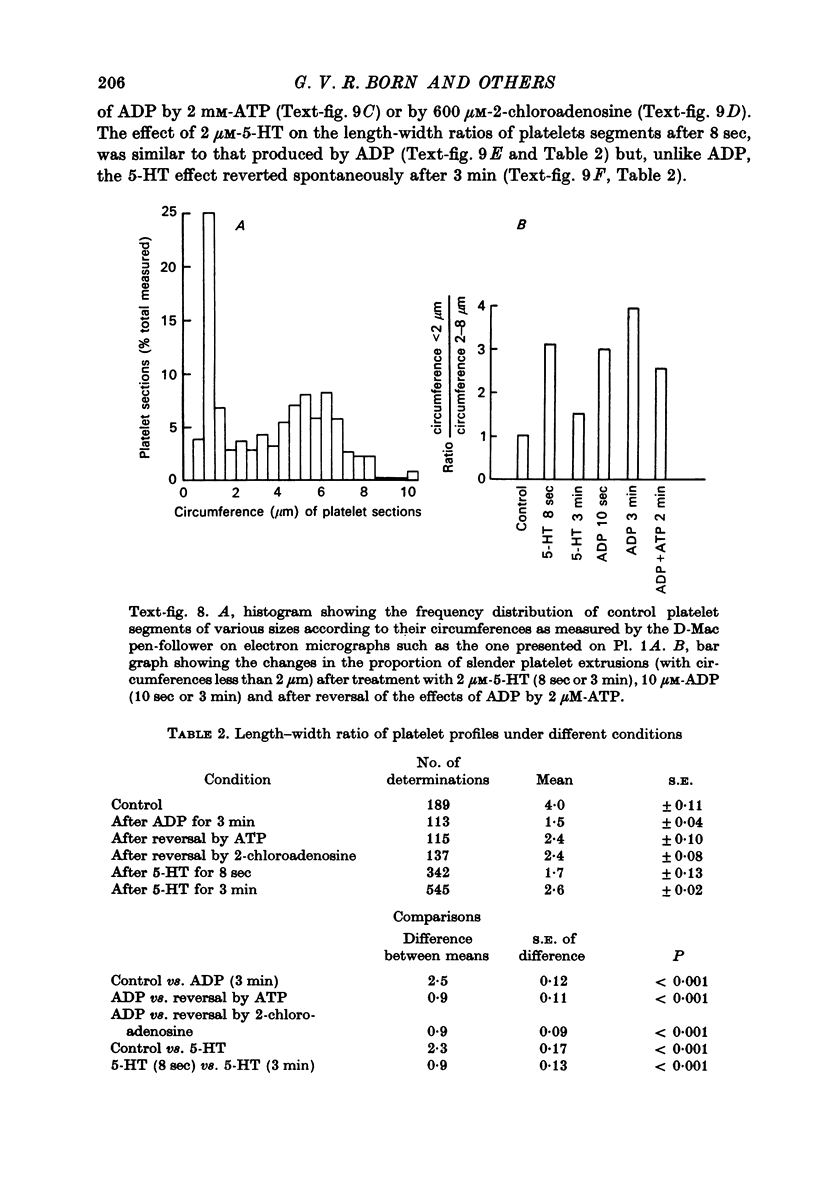
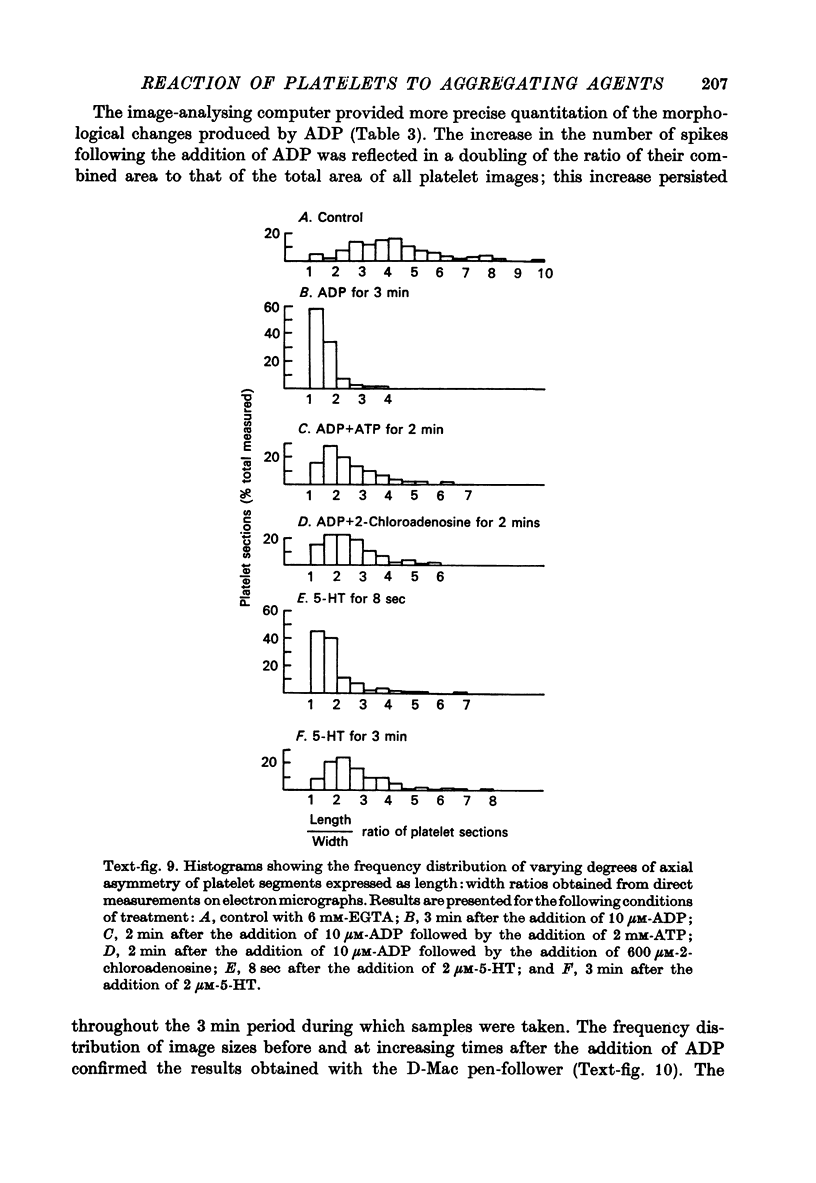
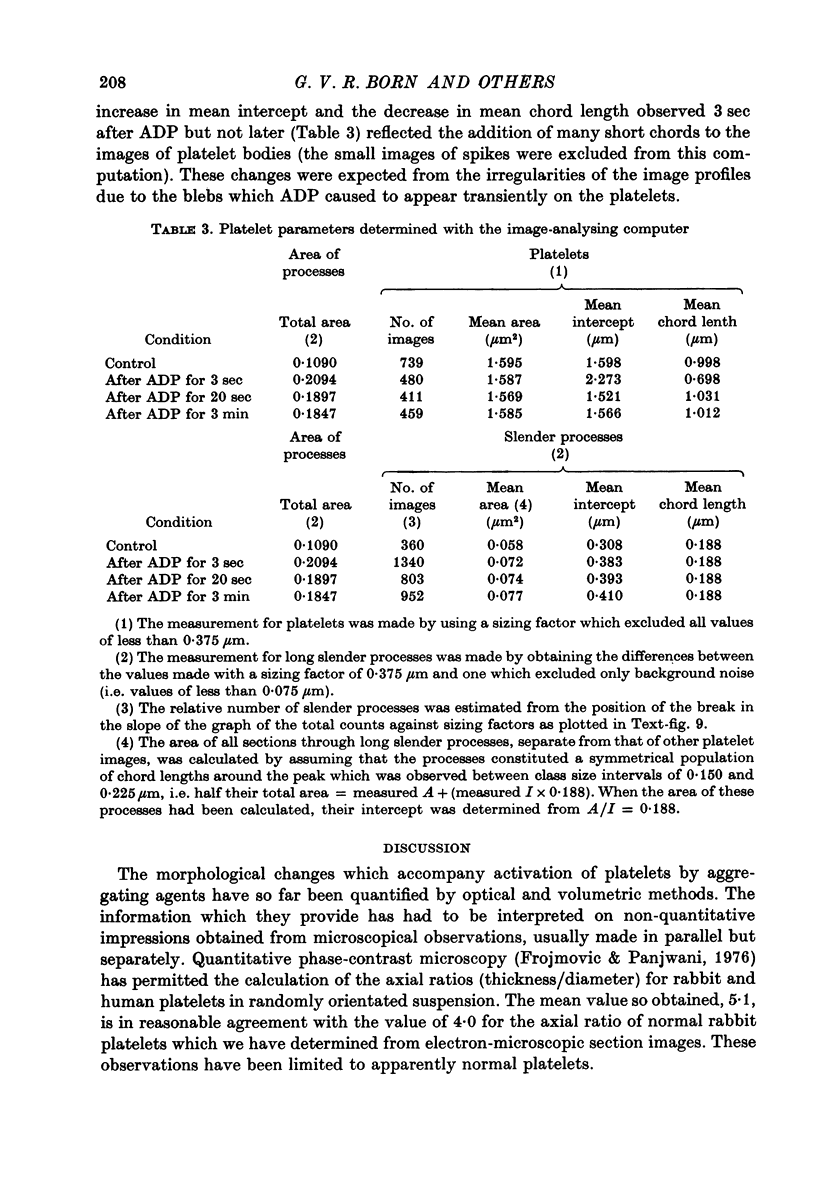
1. In rabbit citrated platelet-rich plasma, the changes in shape of the platelets produced by adenosine diphosphate (ADP) or 5-hydroxytryptamine (5-HT) were observed by photometric and volumetric techniques and by measurements of platelet images on electron micrographs either directly or with an image analysing computer. This permitted the indirect manifestations of the shape changes to be correlated with the morphological features responsible for them, i.e. transformation of disks to more spherical forms and extrusion of blebs and spikes. 2. Following the addition of ADP, an initial brief peak in the light scattering records was associated with marked but transient irregularities in the surface of the platelets. These effects were absent when the other shape changes were produced by 5-HT. 3. When the optical manifestations of the shape changes induced by ADP were reversed by the addition of antagonists adenosine triphosphate, 2-chloroadenosine or prostaglandin E1, the morphological changes were reversed by a diminution in the number of spikes and the conversion of spherical platelet bodies to a more discoid form. 4. The volume of extracellular plasma trapped between platelets sedimented by centrifugation was proportional to the number of spikes which they extruded. Under all conditions, the volume of the platelets themselves remained remarkably constant at approximately 5 X 10(-9) microliter./platelet. 5. Addition of a calcium chelating agent alone produced a rapid persistent alteration in the optical properties of platelet-rich plasma. The magnitude of this alteration was proportional to chelator concentration but greater in some plasmas than in others. Similar optical effects were produced when the calcium concentration of platelet-rich plasma was increased by adding calcium. The optical effects produced by calcium or its chelators were unusual in that the changes in transmitted and horizontally scattered light were in opposite directions (transmitted light decreased, scattered light increased) whereas, in all other circumstances so far examined, these changes were in the same direction. The chelators caused the formation of spikes on the platelets without appreciably altering their disk shape, which may explain the unusual nature of the optical effects.
Full text
PDF



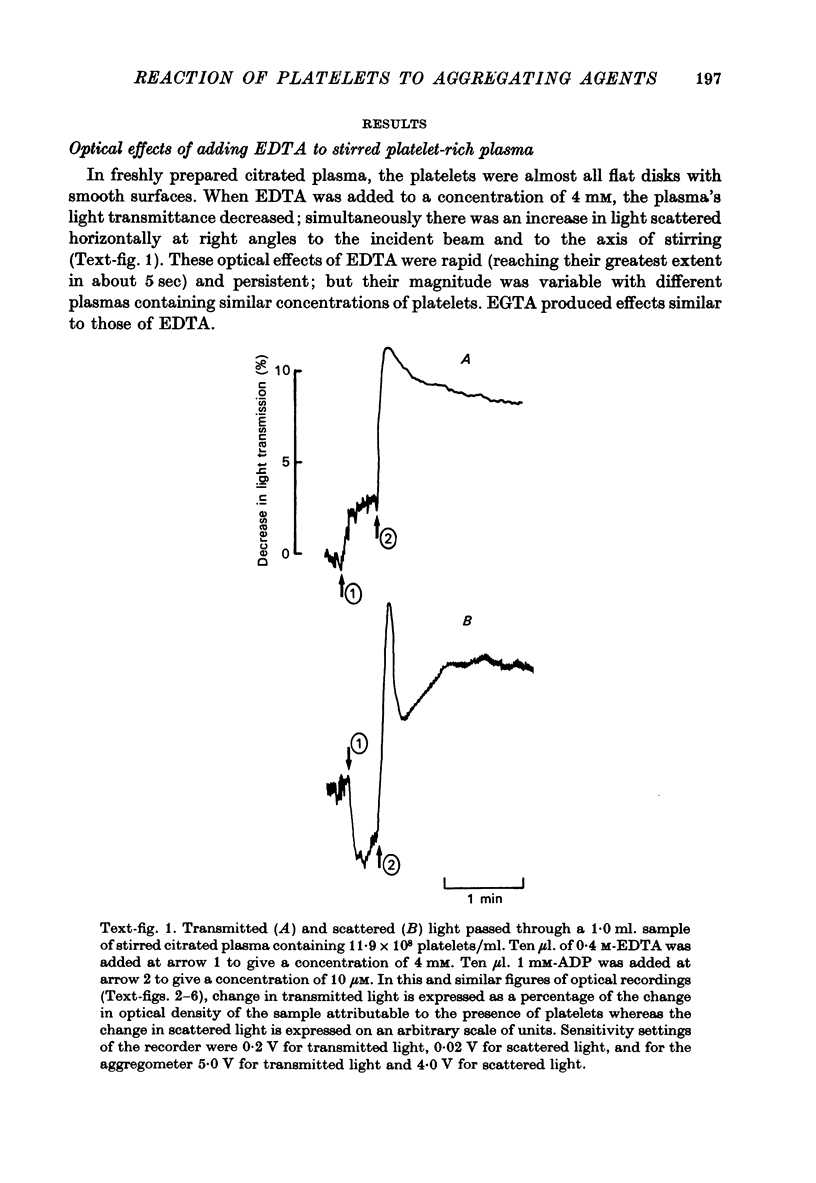
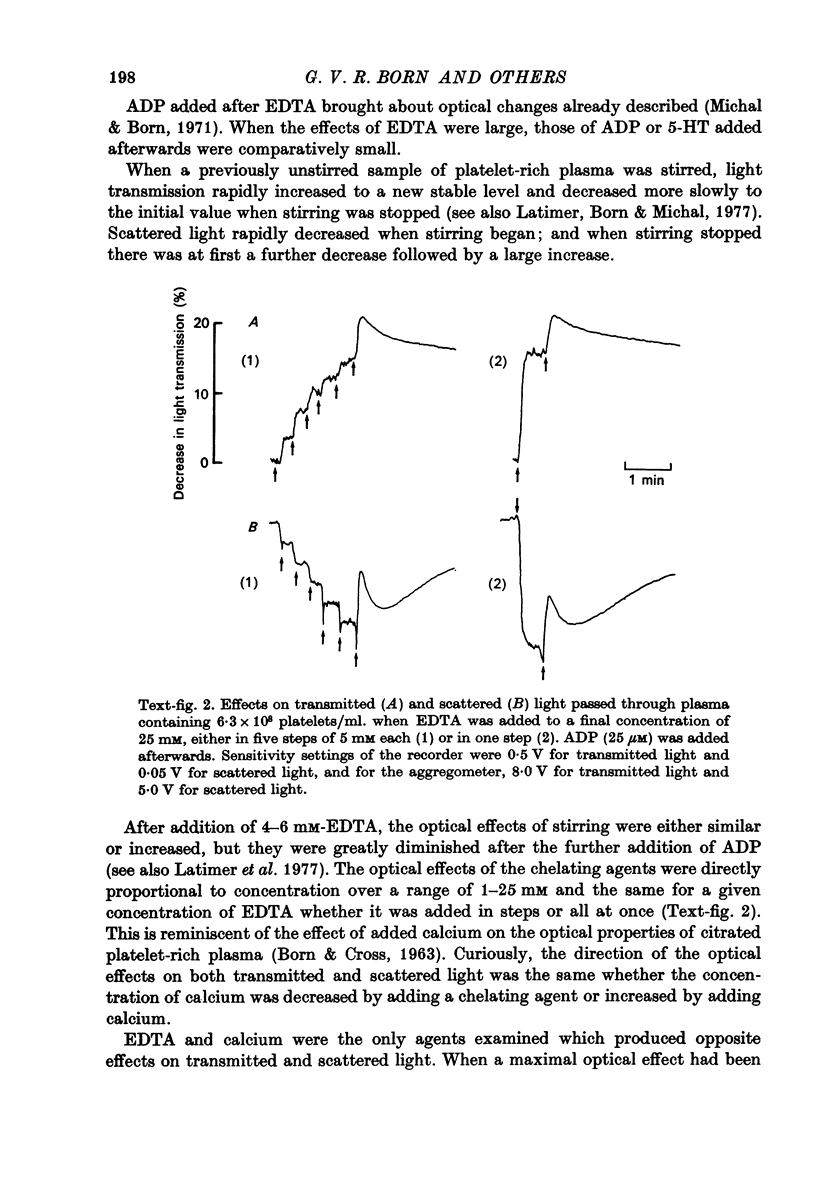
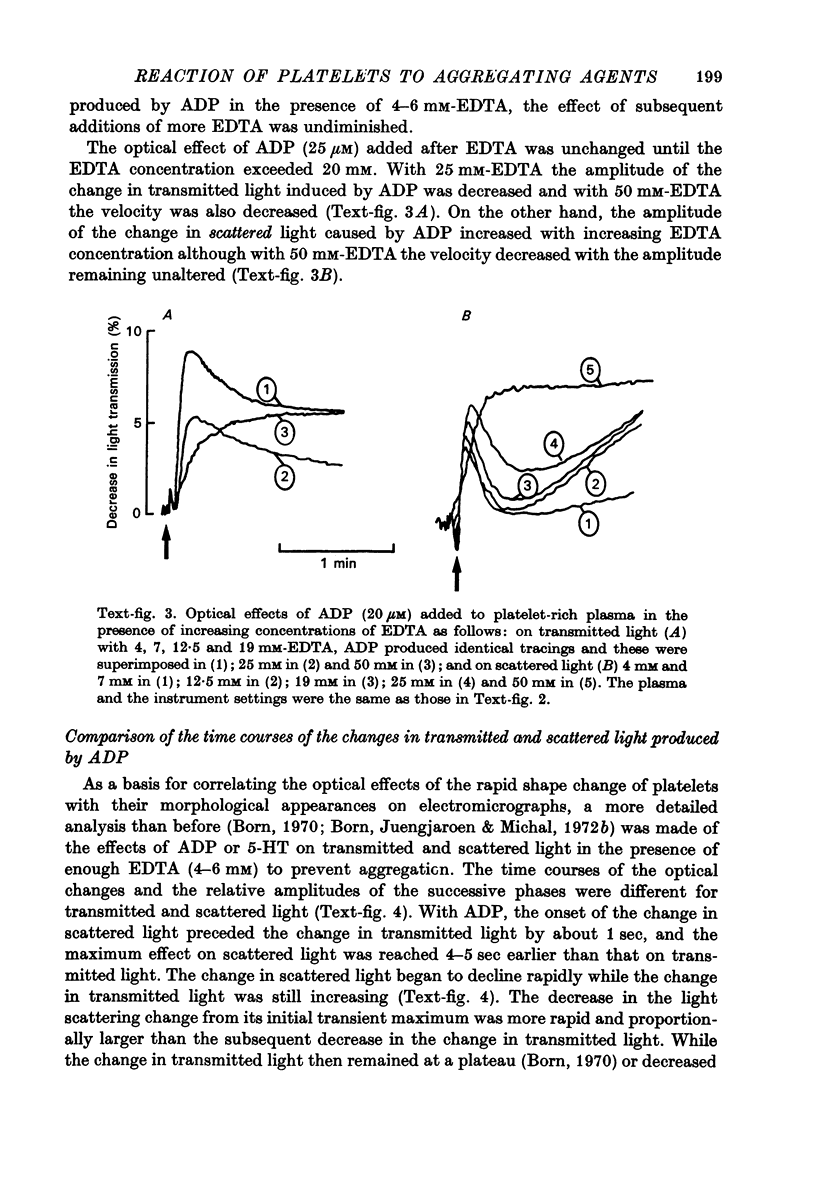
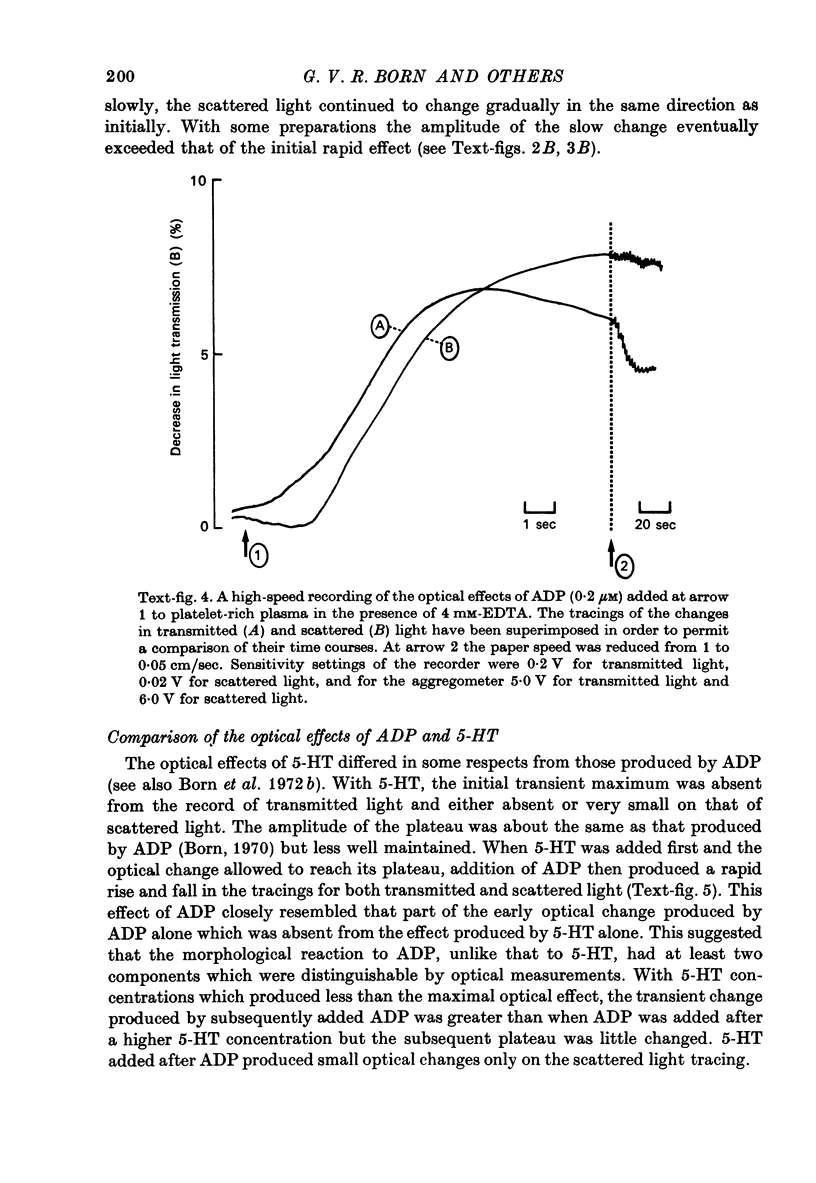
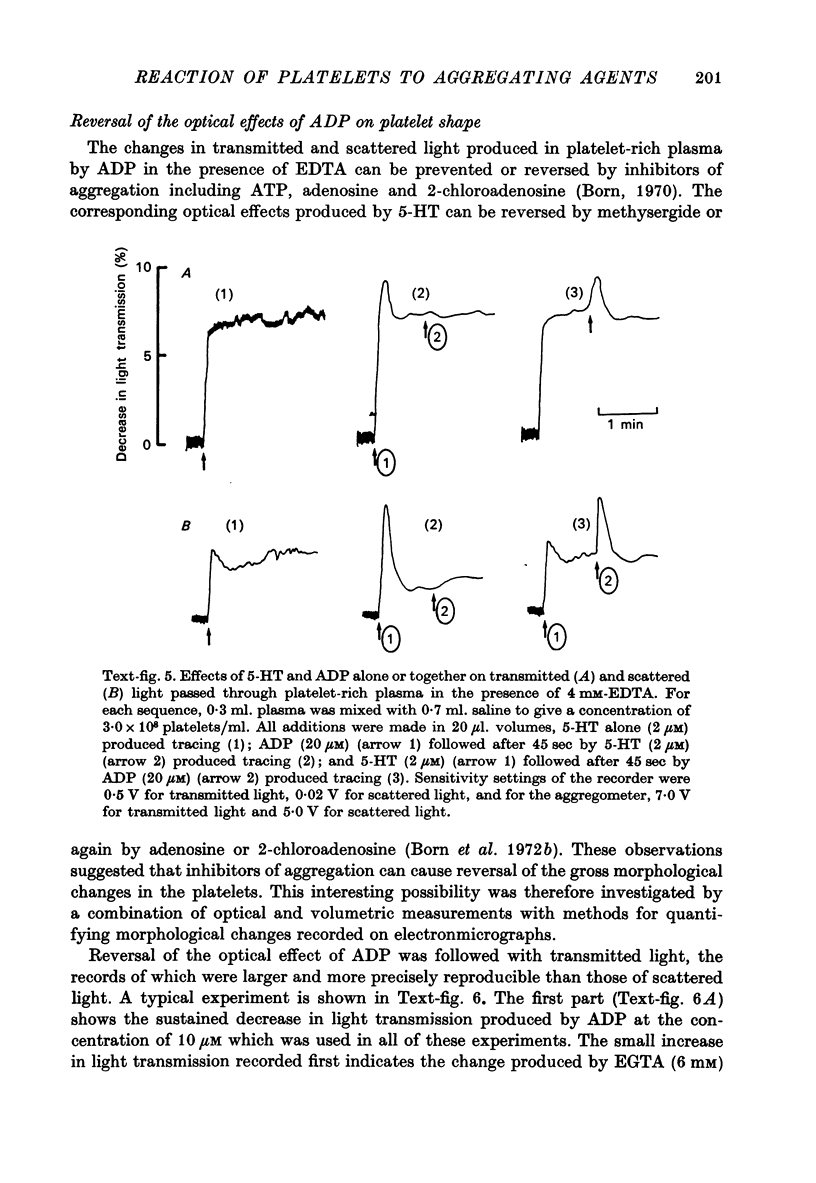
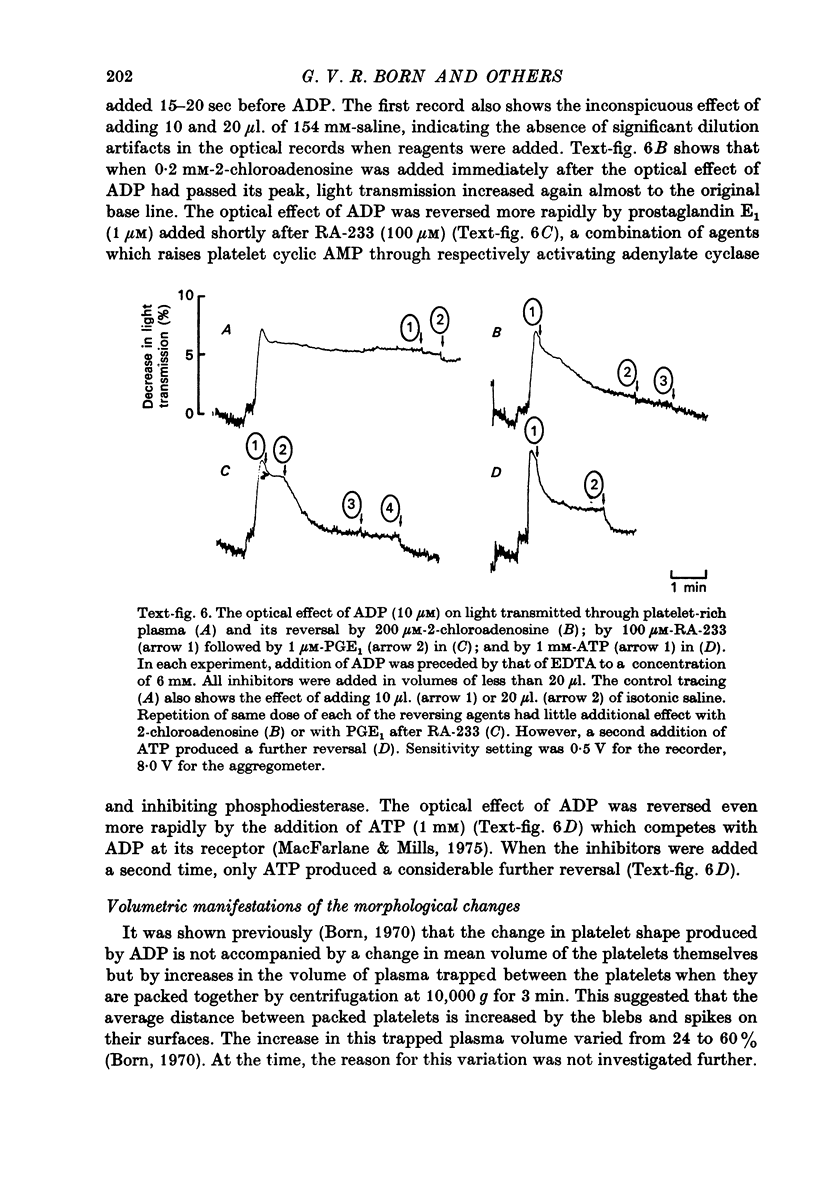
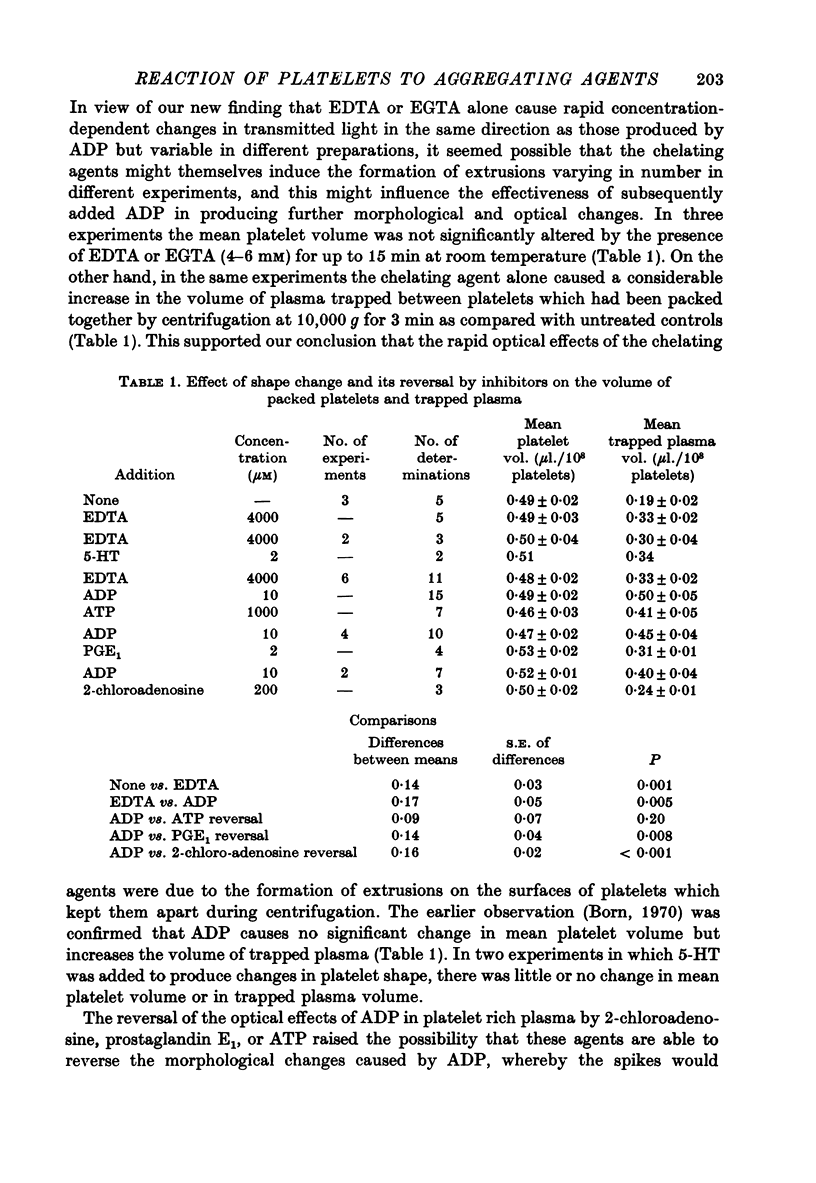









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born G. V., Foulks J., Michal F., Sharp D. E. Reversal of the rapid morphological reaction of platelets. J Physiol. 1972 Sep;225(2):27P–28P. [PubMed] [Google Scholar]
- Born G. V., Juengjaroen K., Michal F. Relative activities on and uptake by human blood platelets of 5-hydroxytryptamine and several analogues. Br J Pharmacol. 1972 Jan;44(1):117–139. doi: 10.1111/j.1476-5381.1972.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born G. V. Mechanism of platelet aggregation and of its inhibition by adenosine derivatives. Fed Proc. 1967 Jan-Feb;26(1):115–117. [PubMed] [Google Scholar]
- Born G. V. Observations on the change in shape of blood platelets brought about by adenosine diphosphate. J Physiol. 1970 Aug;209(2):487–511. doi: 10.1113/jphysiol.1970.sp009176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born G. V. Uptake of adenosine and of adenosine diphosphate by human blood platelets. Nature. 1965 Jun 12;206(989):1121–1122. doi: 10.1038/2061121a0. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M., Panjwani R. Geometry of normal mammalian platelets by quantitative microscopic studies. Biophys J. 1976 Sep;16(9):1071–1089. doi: 10.1016/S0006-3495(76)85756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton E., Gent M., Hirsh J., Harker L. A. Platelet-inhibiting drugs in the prevention of clinical thrombotic disease (third of three parts). N Engl J Med. 1975 Dec 18;293(25):1296–1300. doi: 10.1056/NEJM197512182932506. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Gent M., Genton E. The current status of platelet suppressive drugs in the treatment of thrombosis. Thromb Diath Haemorrh. 1975 Jun 30;33(3):406–416. [PubMed] [Google Scholar]
- Latimer P., Born G. V., Michal F. Application of light-scattering theory to the optical effects associated with the morphology of blood platelets. Arch Biochem Biophys. 1977 Apr 15;180(1):151–159. doi: 10.1016/0003-9861(77)90019-4. [DOI] [PubMed] [Google Scholar]
- MACMILLAN D. C., OLIVER M. F. THE INITIAL CHANGES IN PLATELET MORPHOLOGY FOLLOWING THE ADDITION OF ADENOSINE DIPHOSPHATE. J Atheroscler Res. 1965 Jul-Aug;5(4):440–444. doi: 10.1016/s0368-1319(65)80081-3. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E., Mills D. C. The effects of ATP on platelets: evidence against the central role of released ADP in primary aggregation. Blood. 1975 Sep;46(3):309–320. [PubMed] [Google Scholar]
- McLean J. R., Veloso H. Change of shape without aggregation caused by ADP in rabbit platelets at low pH. Life Sci. 1967 Sep 15;6(18):1983–1986. doi: 10.1016/0024-3205(67)90259-7. [DOI] [PubMed] [Google Scholar]
- Michal F., Born G. V. Effect of the rapid shape change of platelets on the transmission and scattering of light through plasma. Nat New Biol. 1971 Jun 16;231(24):220–222. doi: 10.1038/newbio231220a0. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Commentary. Drugs inhibiting platelet function. Biochem Pharmacol. 1973 Dec 15;22(24):3151–3156. doi: 10.1016/0006-2952(73)90089-0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard B. L. The effect of acetylsalicylic acid on platelet adhesion in the injured abdominal aorta. Q J Exp Physiol Cogn Med Sci. 1972 Jul;57(3):319–323. doi: 10.1113/expphysiol.1972.sp002165. [DOI] [PubMed] [Google Scholar]
- Sherry S. Clinical trials with antithrombotic and thrombolytic agents. Principles and pitfalls. Thromb Diath Haemorrh. 1973 Feb 28;29(1):3–10. [PubMed] [Google Scholar]