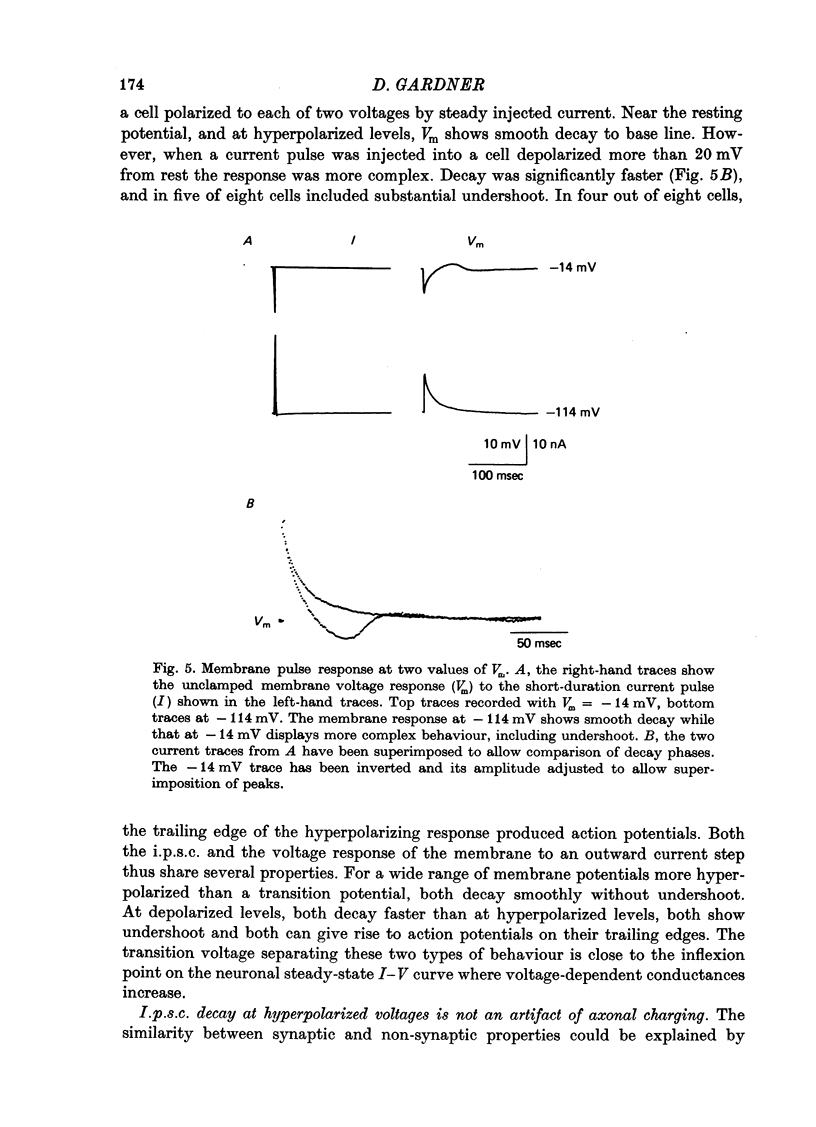
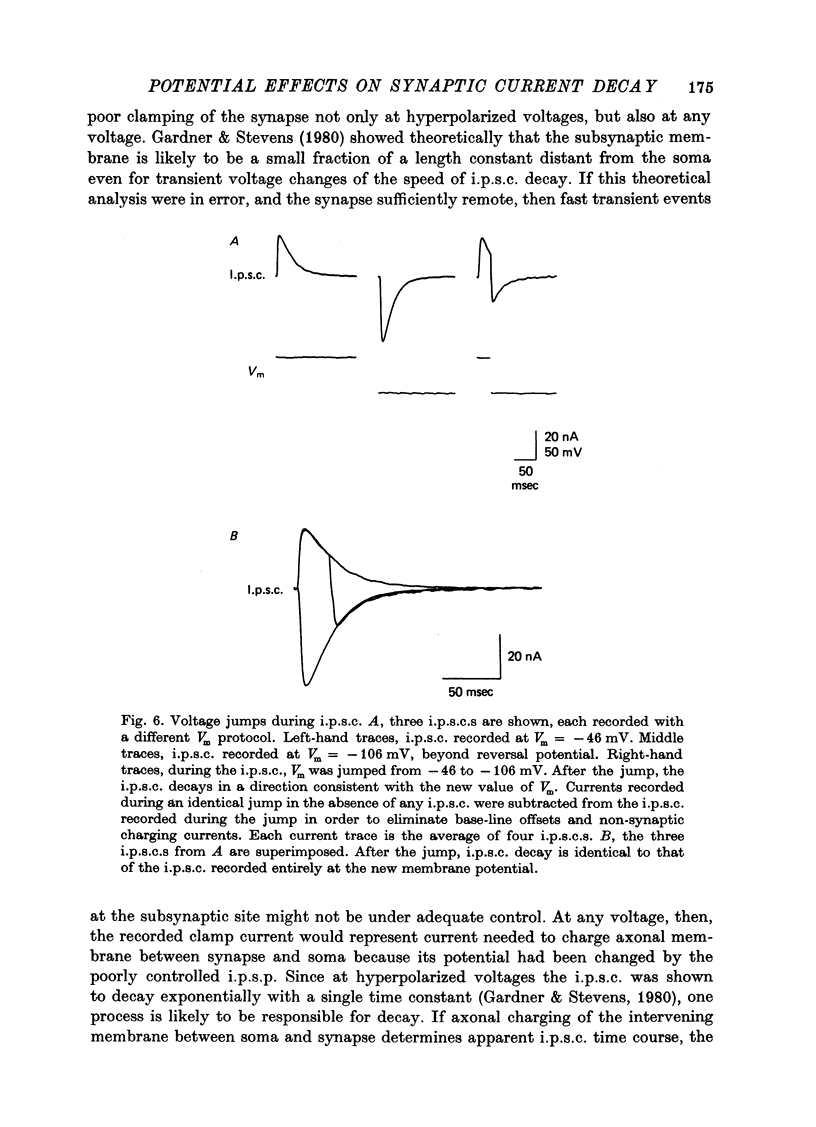
Abstract
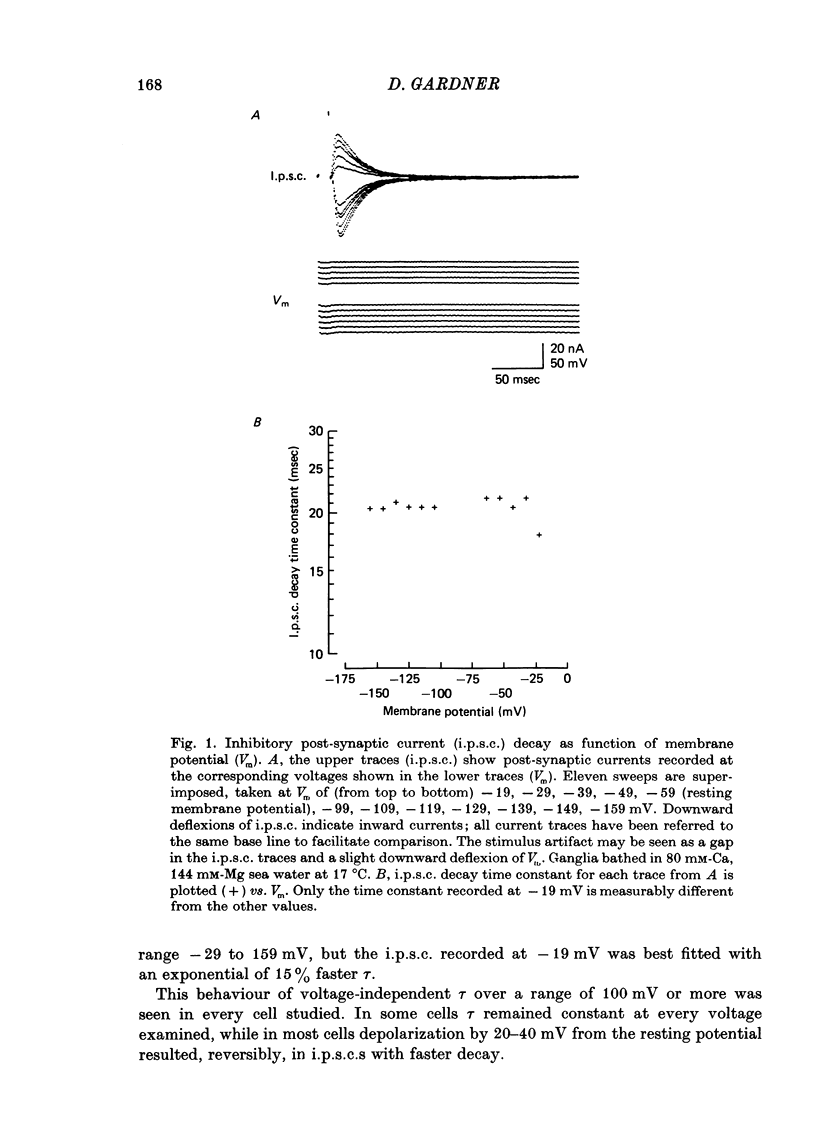
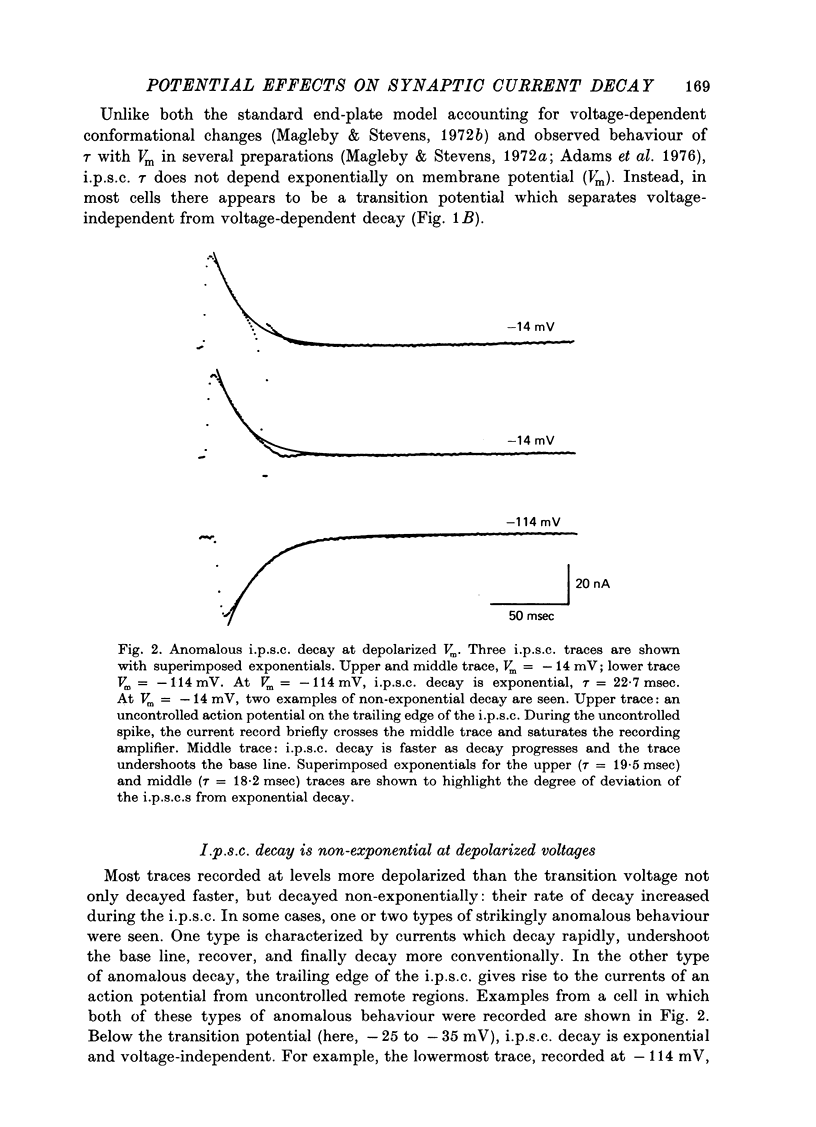
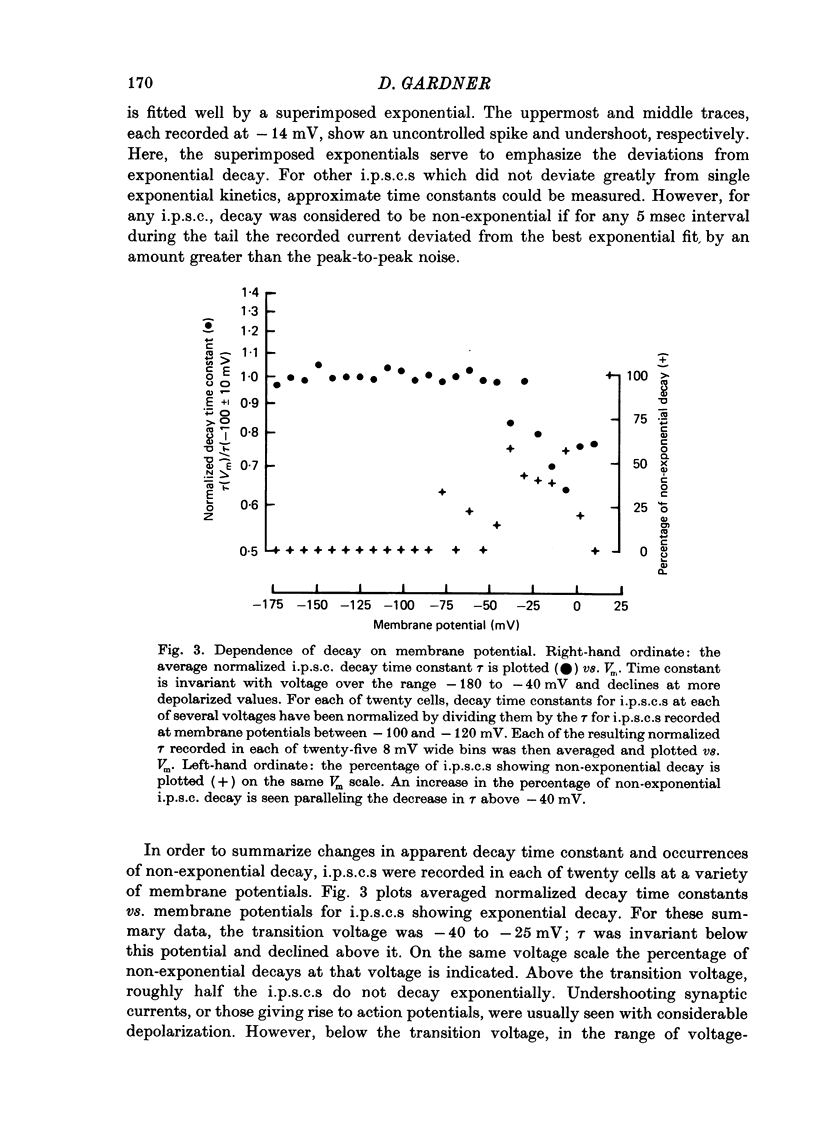
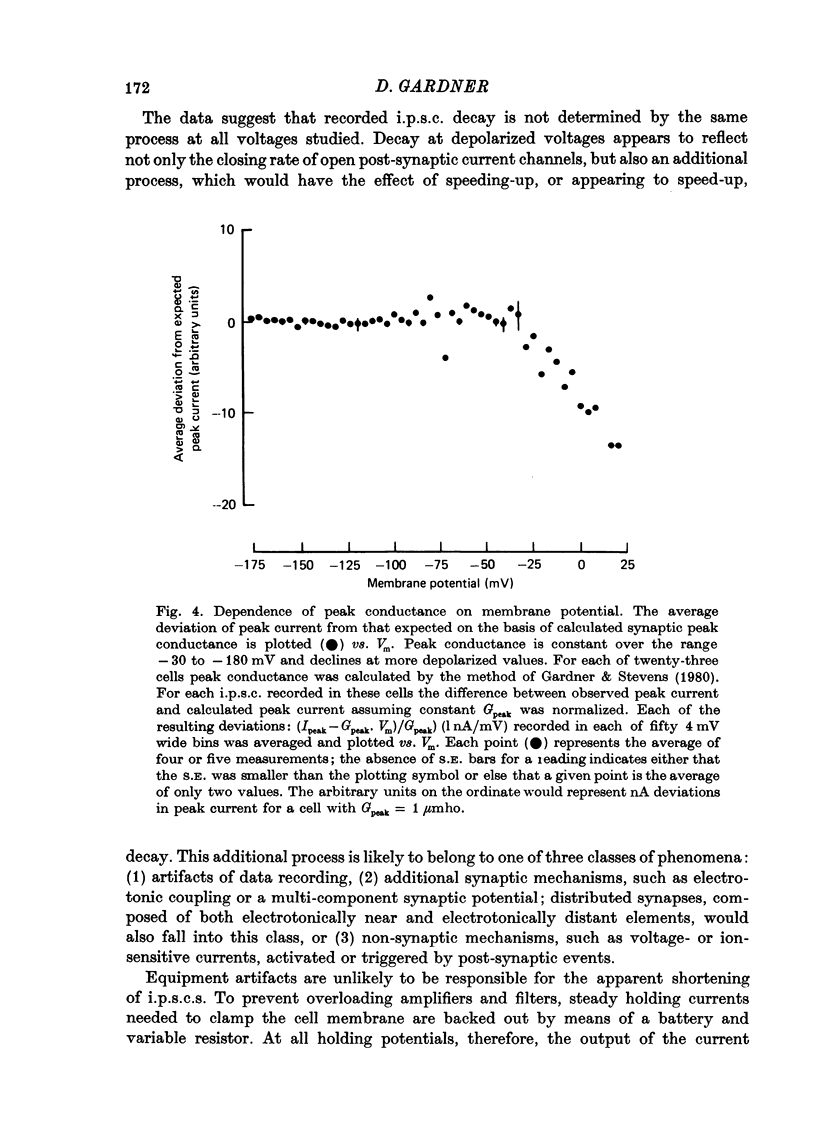
1. Inhibitory post-synaptic currents (i.p.s.c.s) were recorded under voltage clamp using two electrodes placed in neuronal somas of the buccal ganglia of Aplysia, in order to study the effects of membrane potential (Vm) on decay time constant (tau). 2. From -175 to -40 mV, tau did not vary with Vm. At Vm more depolarized than -40 mV, tau decreased. Also at depolarized Vm, cell input resistance (Rin) decreased and many cells showed non-exponential i.p.s.c. decay, including undershoot. These results suggest that the apparently faster tau is an artifact of remote membrane poorly clamped at the low Rin of depolarized levels, rather than a Vm-dependent i.p.s.c. relaxation. 3. Injected current pulses produced voltage relaxations which decayed faster including undershoot, when Vm was depolarized beyond -40 mV. 4. Step commands across the reversal potential were delivered during i.p.s.c.s. Currents reversed direction, relaxing consistent with the new Vm, thus showing that recorded current decay repressants the true time course of i.p.s.c. relaxation, rather than uncontrolled slow axonal charging from a fast remote synaptic current. 5. I conclude that clamp control is poor at depolarized Vm, due to decreased Rin, and the faster, non-exponential decay seen includes a superimposed nonsynaptic current. Tetraethylammonium injected into the presynaptic neurone produces only slight effect on the i.p.s.c. decay time constant, suggesting that the non-synaptic current is unlikely to be due to a voltage-dependent K conductance.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W., Hamill O. P. Voltage sensitivity of inhibitory postsynaptic current in Aplysia buccal ganglia. Brain Res. 1976 Oct 22;115(3):506–511. doi: 10.1016/0006-8993(76)90368-1. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate and quisqualate noise in voltage-clamped locust muscle fibres. Nature. 1976 May 13;261(5556):151–153. doi: 10.1038/261151a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Armstrong C. M. Miniature end-plate currents in voltage-clamped muscle fibre. Nature. 1968 Apr 27;218(5139):363–365. doi: 10.1038/218363b0. [DOI] [PubMed] [Google Scholar]
- Gardner D., Stevens C. F. Rate-limiting step of inhibitory post-synaptic current decay in Aplysia buccal ganglia. J Physiol. 1980 Jul;304:145–164. doi: 10.1113/jphysiol.1980.sp013316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Inhibitory postsynaptic current in voltage-clamped crayfish muscle. Nature. 1976 Sep 9;263(5573):153–154. doi: 10.1038/263153a0. [DOI] [PubMed] [Google Scholar]
- Pellmar T. C., Wilson W. A. Unconventional serotonergic excitation in Aplysia. Nature. 1977 Sep 1;269(5623):76–78. doi: 10.1038/269076a0. [DOI] [PubMed] [Google Scholar]


