Abstract
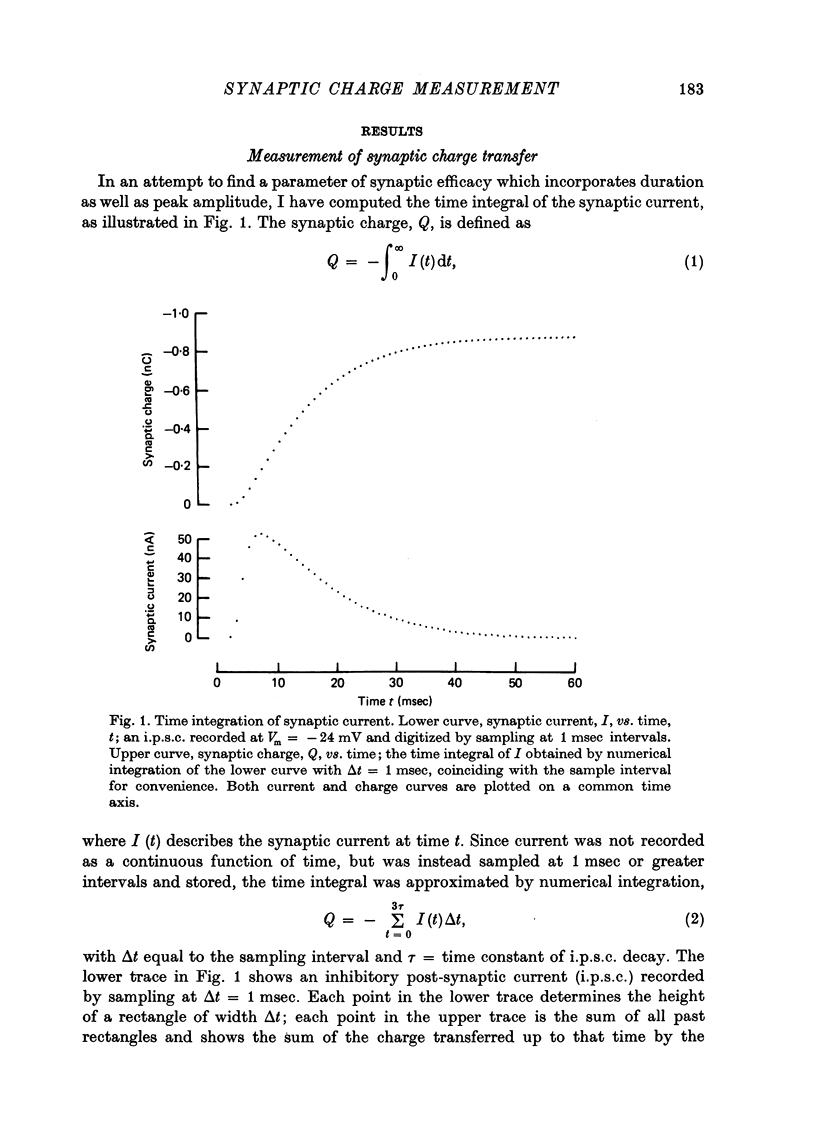
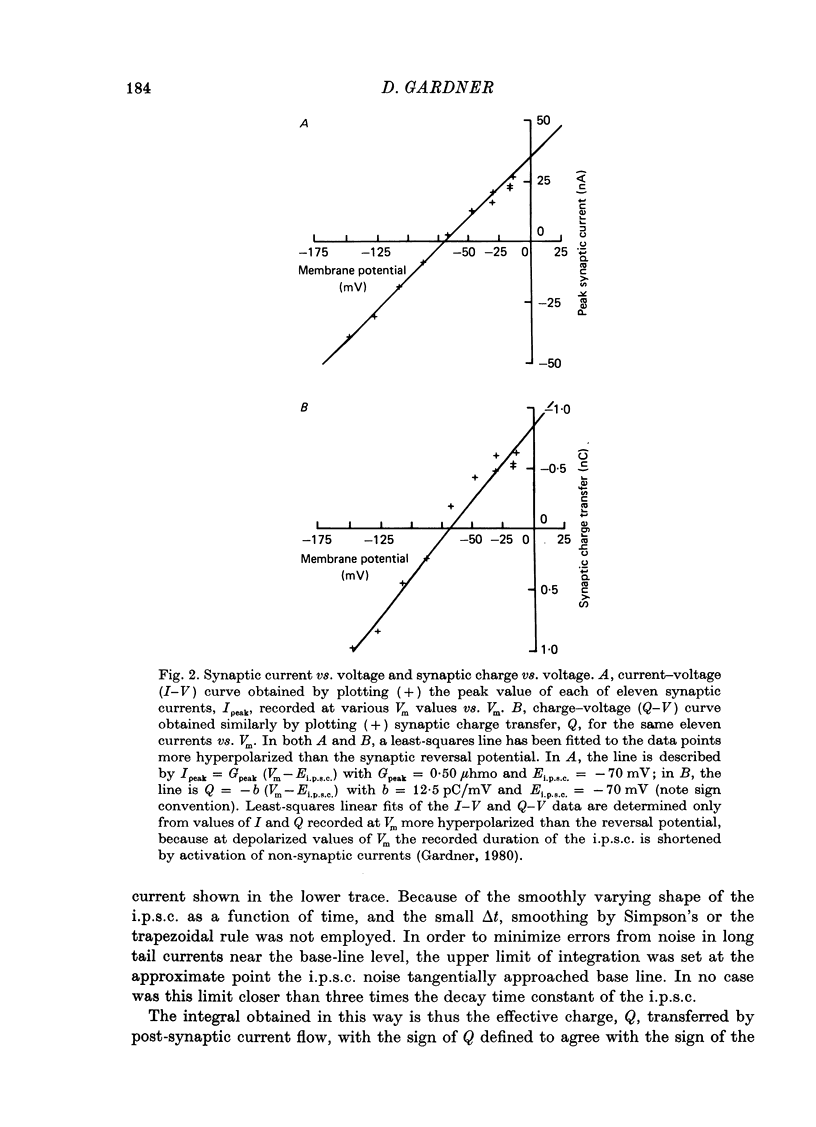
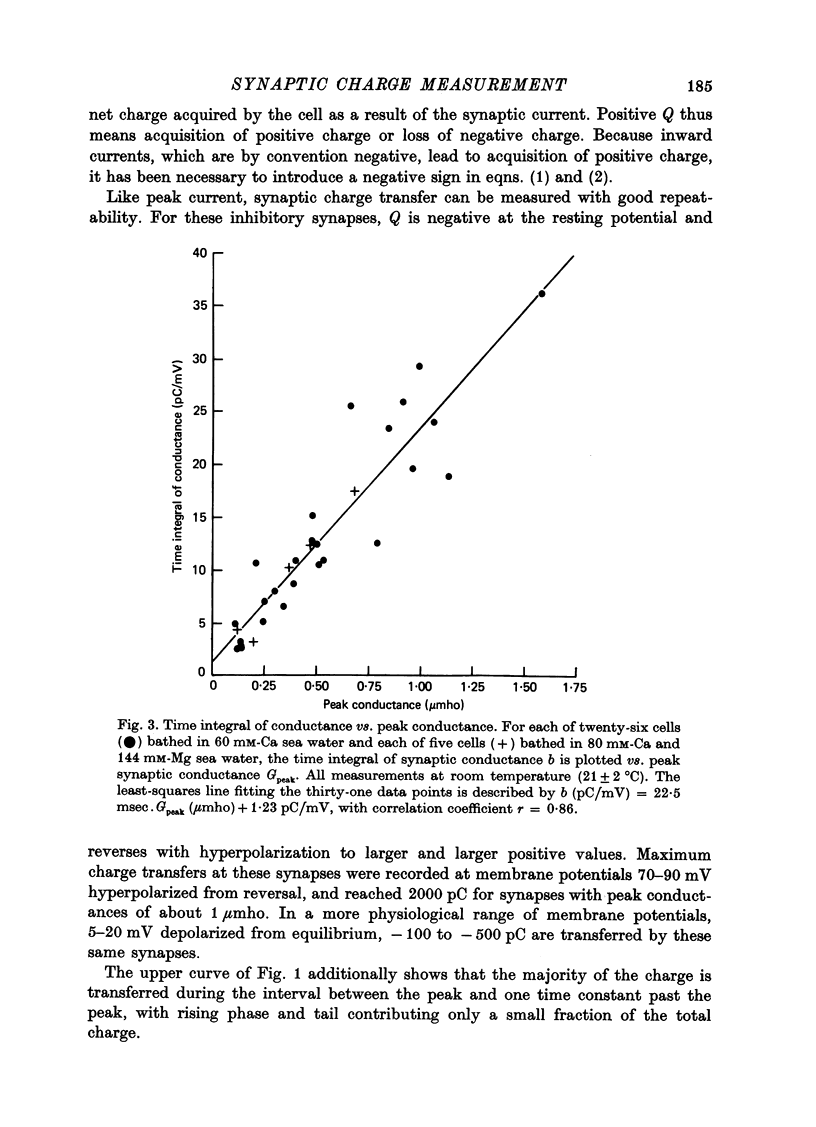
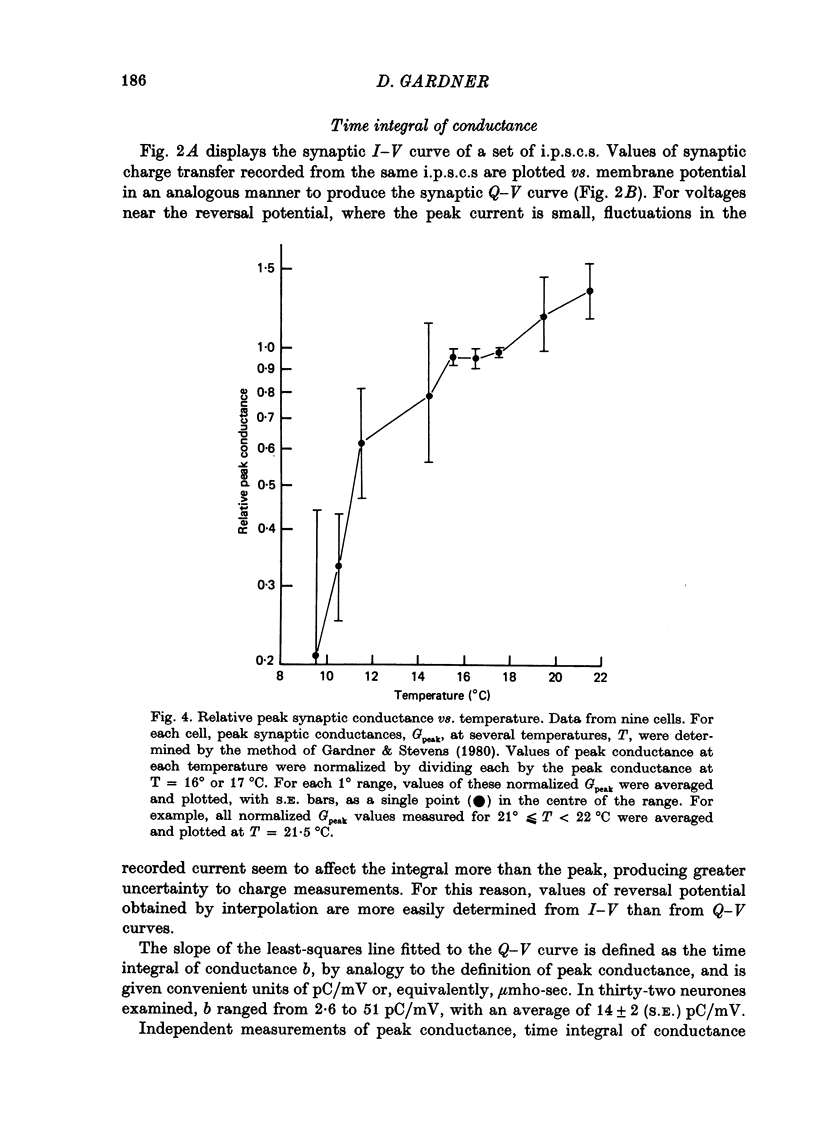
1. Inhibitory post-synaptic currents (i.p.s.c.s) were recorded under voltage clamp from neurones of Aplysia buccal ganglia. 2. The synaptic charge, Q, transferred by each i.p.s.c. was calculated as the time integral of the synaptic current, approximated by numerical integration. For typical i.p.s.c.s recorded at or near resting potential, Q = -100 to -500 pC. The majority of the charge is transferred during the interval between the peak and a time one time constant later. 3. In order to characterize an alternative measurement of synaptic efficacy, the slope of the Q vs. membrane potential curve was calculated and defined as the time integral of conductance, b. Values of b ranged from 2.6 to 51 pC/mV, averaging 14 pC/mV. For i.p.s.c.s recorded in thirty-one cells at room temperature, b was well correlated with Gpeak, the peak synaptic conductance (r = 0.86). 4. In most synapses, the time integral of conductance, which incorporates both amplitude and duration, may be a more revealing measure of synaptic efficacy than peak conductance. 5. Size of synaptic response was determined as a function of temperature, T. While Gpeak decreases with decreasing temperature over the range 9-22 degrees C, b peaks at 12-18 degrees C and decreases at higher and lower values of T. The data permit the speculation that lengthening average channel lifetime, and therefore time constant of decay, with decreasing temperature, may have adaptive significance in maintaining synaptic efficacy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Cull-Candy S. G., Miledi R. Potential-dependent transition temperature of ionic channels induced by glutamate in locust muscle. Nature. 1977 Aug 18;268(5621):663–665. doi: 10.1038/268663a0. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Influence of dendritic location and membrane properties on the effectiveness of synapses on cat motoneurones. J Physiol. 1974 Jun;239(2):325–345. doi: 10.1113/jphysiol.1974.sp010571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H. Dendritic synapses and reversal potentials: theoretical implications of the view from the soma. Exp Neurol. 1969 Jun;24(2):248–264. doi: 10.1016/0014-4886(69)90018-1. [DOI] [PubMed] [Google Scholar]
- Dudel J. Potentiation and desensitization after glutamate induced postsynaptic currents at the crayfish neuromuscular junction. Pflugers Arch. 1975;356(4):317–327. doi: 10.1007/BF00580005. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. Interconnections of identified multiaction interneurons in buccal ganglia of Aplysia. J Neurophysiol. 1977 Mar;40(2):349–361. doi: 10.1152/jn.1977.40.2.349. [DOI] [PubMed] [Google Scholar]
- Gardner D. Membrane-potential effects on an inhibitory post-synaptic conductance in Aplysia buccal ganglia. J Physiol. 1980 Jul;304:165–180. doi: 10.1113/jphysiol.1980.sp013317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D., Stevens C. F. Rate-limiting step of inhibitory post-synaptic current decay in Aplysia buccal ganglia. J Physiol. 1980 Jul;304:145–164. doi: 10.1113/jphysiol.1980.sp013316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ger B. A., Zeimal E. V. Pharmacological study of two kinds of cholinoreceptors on the membrane of identified completely isolated neurones of Planorbarius corneus. Brain Res. 1977 Jan 31;121(1):131–139. doi: 10.1016/0006-8993(77)90443-7. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Rinzel J., Rall W. Transient response in a dendritic neuron model for current injected at one branch. Biophys J. 1974 Oct;14(10):759–790. doi: 10.1016/S0006-3495(74)85948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]


