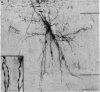
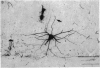
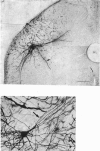
Abstract
1. Intracellular injections of horseradish peroxidase were made in a functionally identified population of motoneurones in spinal cords of cats. These motoneurones were activated by tactile stimulation of the hind-limb central foot pad. 2. Cell bodies of twenty-two such motoneurones were located in the dorsolateral portion of the ventral horn in the first sacral segment. The mean diameter of the major axis of transverse sections through twelve of these cell bodies was 68 . 2 micrometer, the mean diameter of the minor axis was 48 . 7 micrometer. The major axis tended to be oriented dorsomedially-ventrolaterally. 3. In the transverse plane, the dendrites had a characteristic configuration, with a prominent group of dendrites travelling from the cell body dorsomedially into the dorsal horn, entering Rexed's lamina VI. For seventeen motoneurones with well stained dendrites, the mean medial spread of the dendrites was 960 micrometer. Though the mean lateral spread was only 508 micrometer, all of these motoneurones sent dendritic projections into the lateral white matter. The mean dorsal spread of the dendrites was 693 micrometer, the mean ventral spread, 748 micrometer. In the rostrocaudal direction, the mean spread rostrally was 911 micrometer, the mean spread caudally was 998 micrometer. The maximum dendritic spread for a single motoneurone was 2,940 micrometer, in the rostro caudal direction. The sum of dendritic lengths over an entire dendritic tree for the best-stained motoneurones exceeded 13,000 micrometer. 4. The mean diameter of the initial segment of axons of nineteen motoneurones was 4 . 3 micrometer. These axons were notable for the lack or paucity of axon collaterals. Only five of twenty-one axons possessed collaterals; of these, only one possessed more than a single collateral system. This sparseness of the collateral system was reflected in a low level of recurrent inhibition. 5. A possible relationship is discussed between the prominent dorsomedially oriented dendritic bundles of the motoneurones and the axon collaterals of dorsal horn cells mediating cutaneous stimulation which can activate these motoneurones.
Full text
PDF



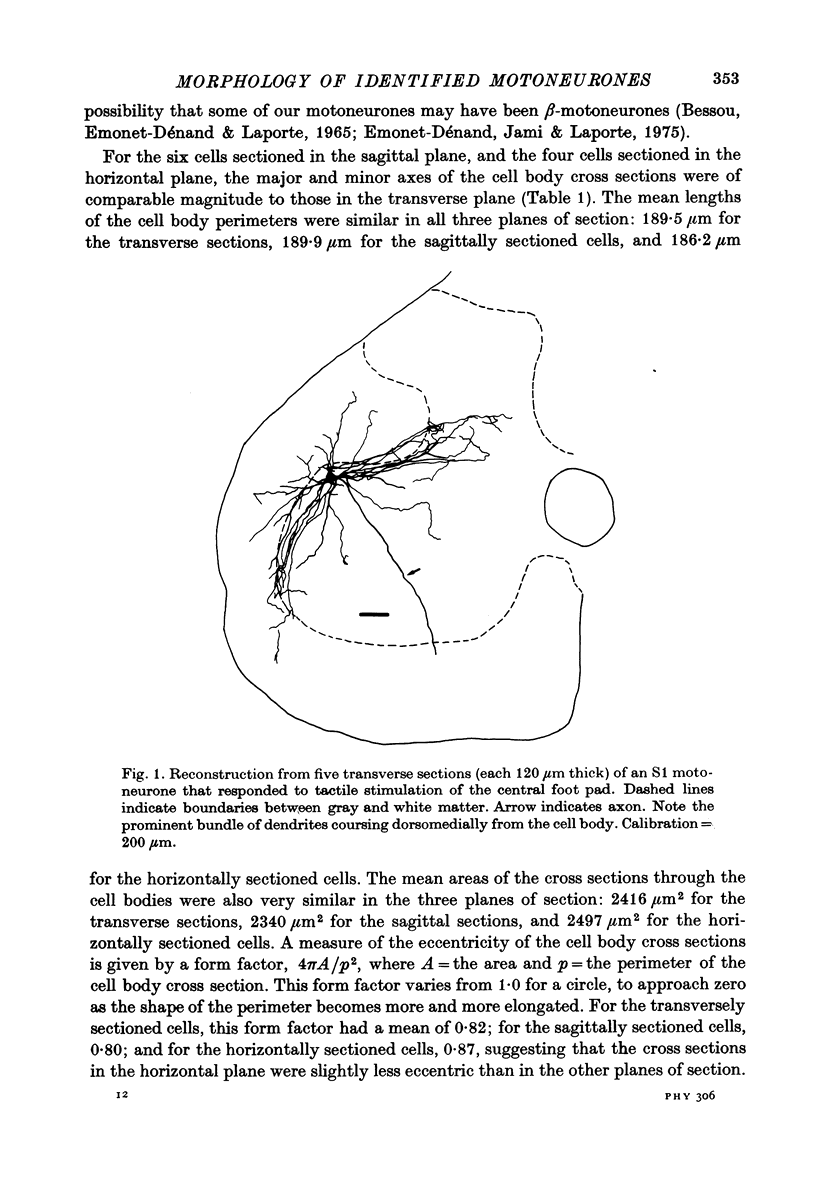
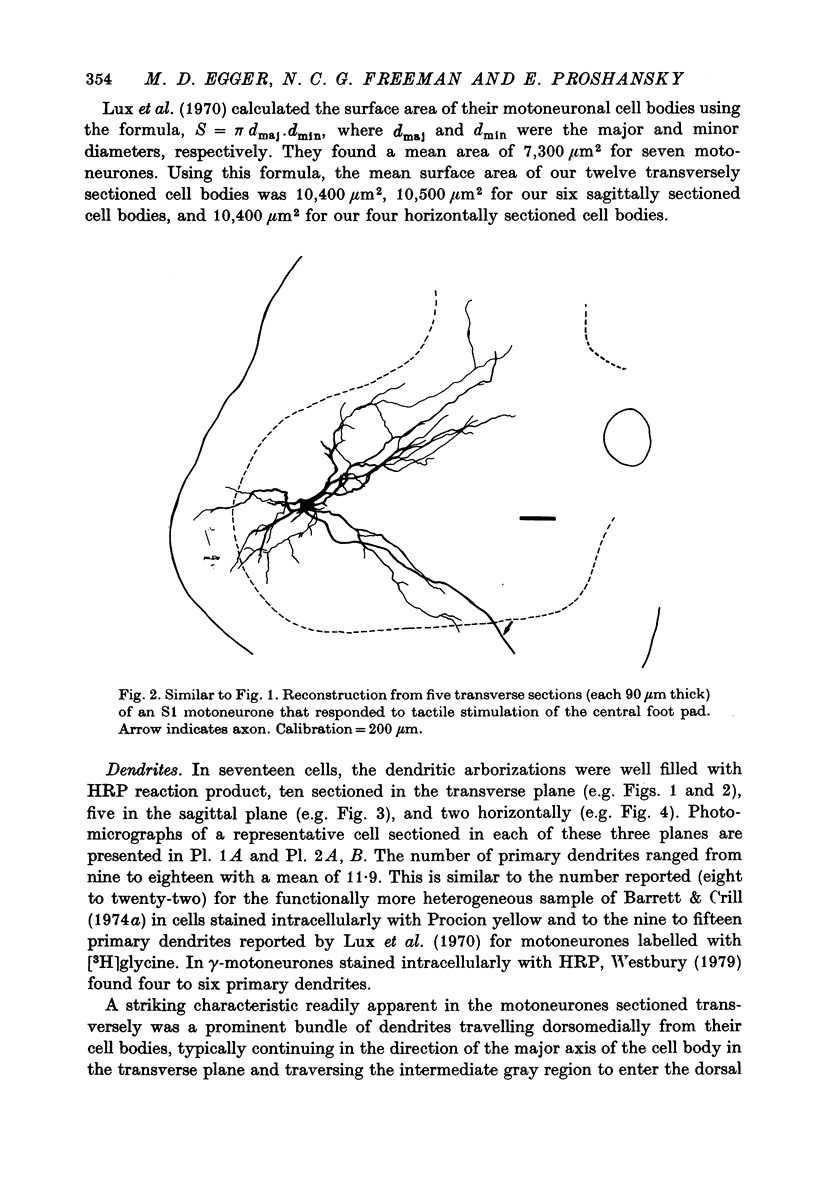

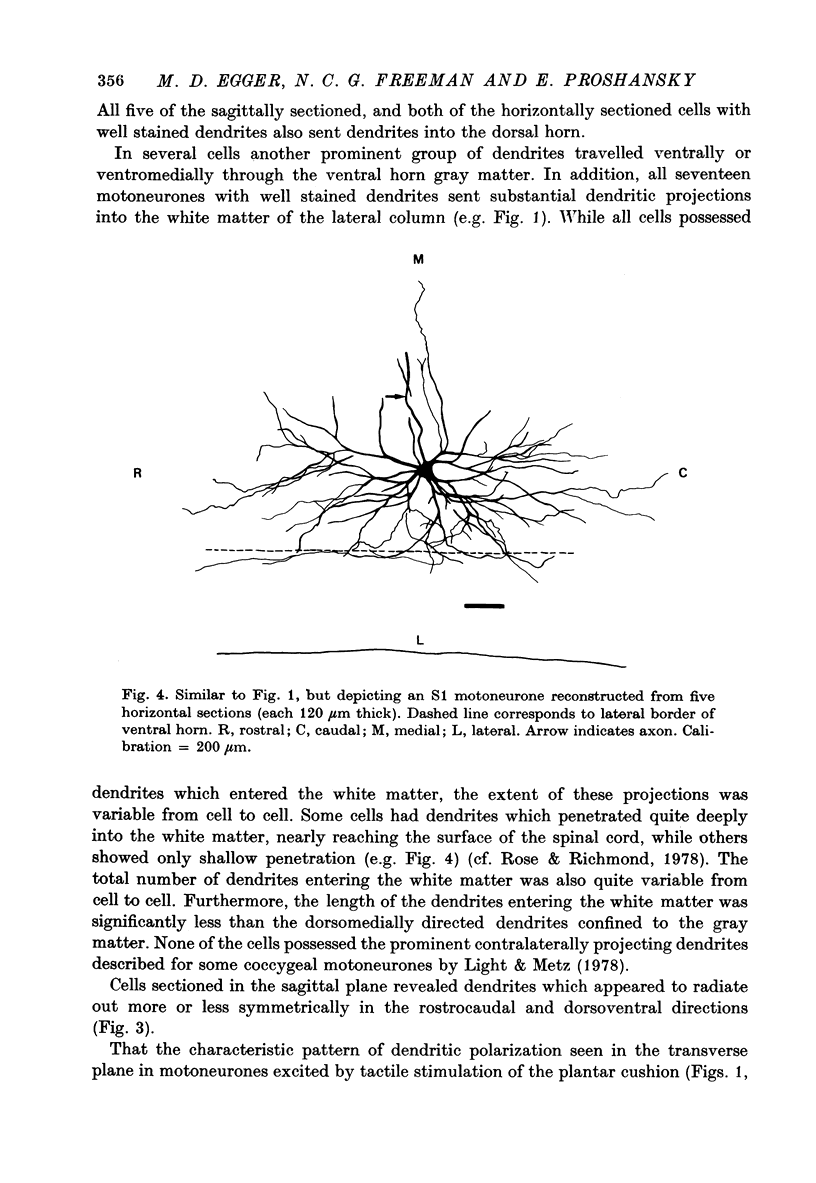
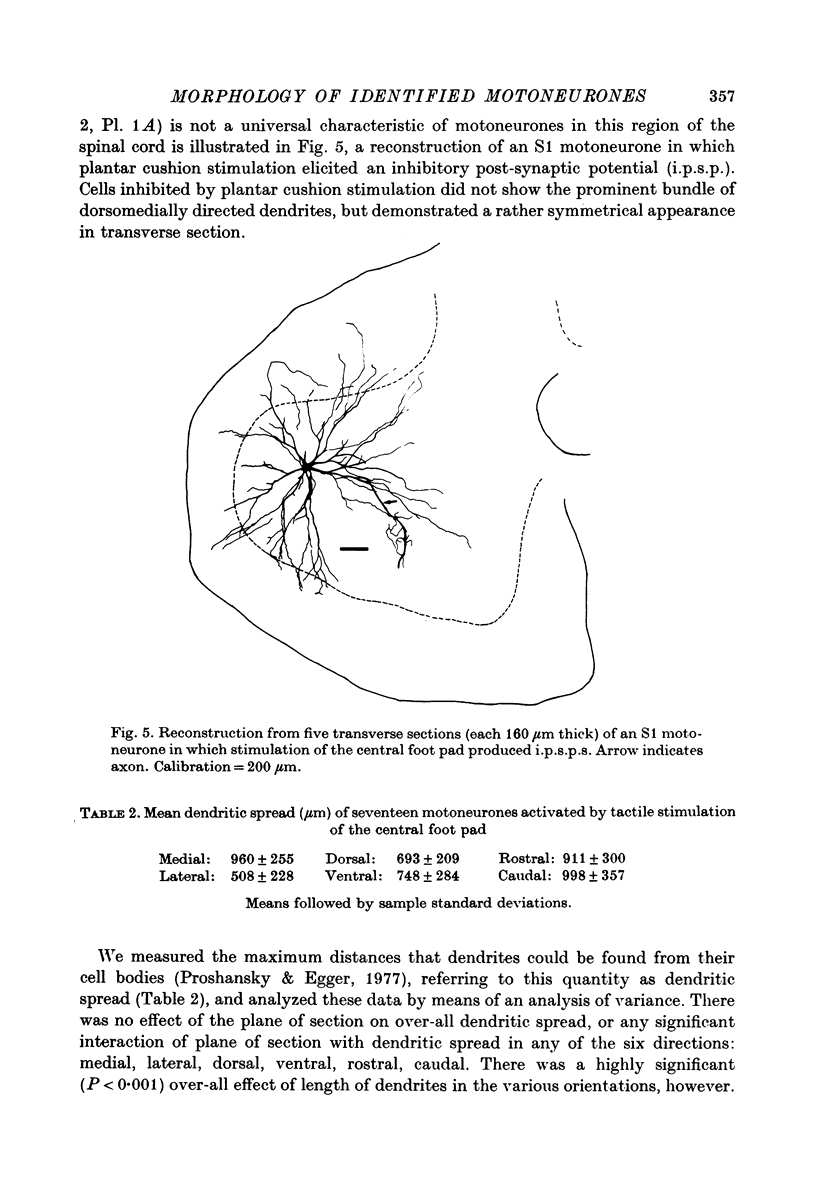

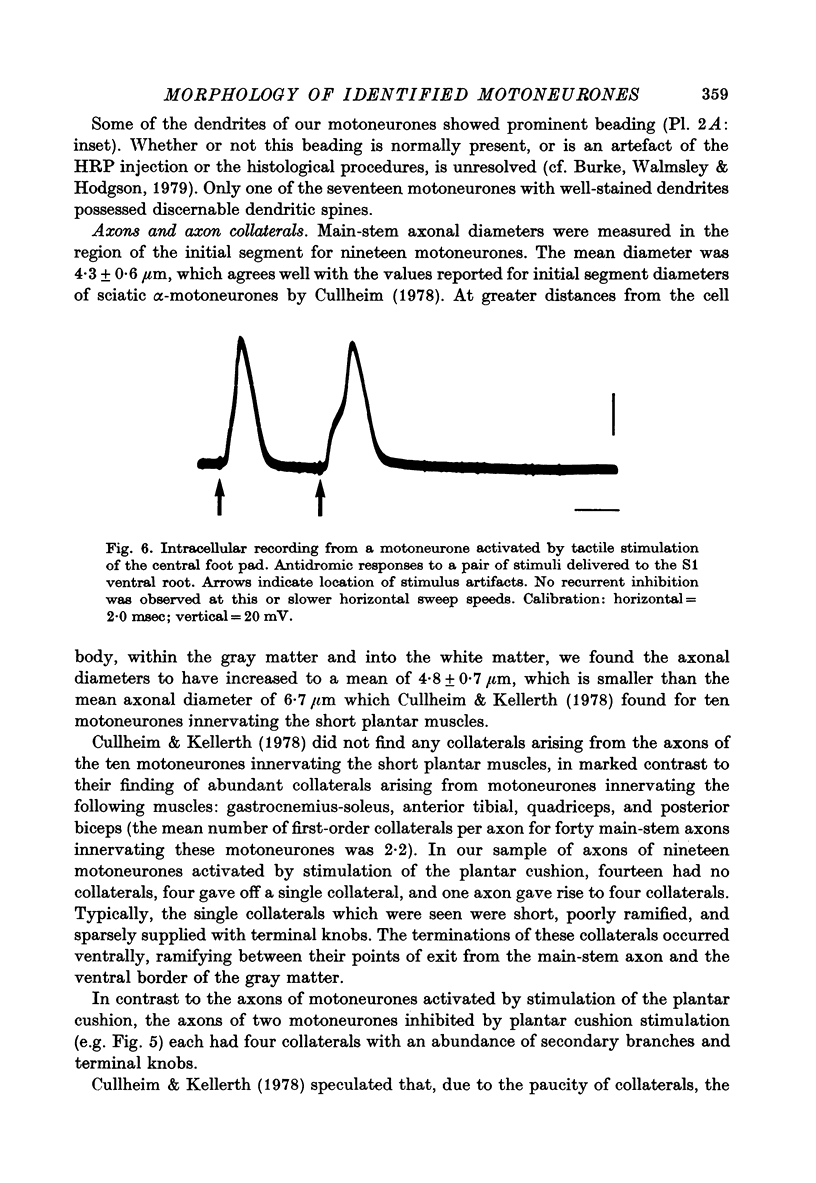






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AITKEN J. T., BRIDGER J. E. Neuron size and neuron population density in the lumbosacral region of the cat's spinal cord. J Anat. 1961 Jan;95:38–53. [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Influence of dendritic location and membrane properties on the effectiveness of synapses on cat motoneurones. J Physiol. 1974 Jun;239(2):325–345. doi: 10.1113/jphysiol.1974.sp010571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Specific membrane properties of cat motoneurones. J Physiol. 1974 Jun;239(2):301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Emonet-Dénand F., Laporte Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J Physiol. 1965 Oct;180(3):649–672. doi: 10.1113/jphysiol.1965.sp007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. N., Trevino D. L., Willis W. D. Evidence for a common location of alpha and gamma motoneurons. Brain Res. 1972 Mar 10;38(1):193–196. doi: 10.1016/0006-8993(72)90602-6. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Walmsley B., Hodgson J. A. HRP anatomy of group Ia afferent contacts on alpha motoneurones. Brain Res. 1979 Jan 12;160(2):347–352. doi: 10.1016/0006-8993(79)90430-x. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different hind-limb muscles. J Physiol. 1978 Aug;281:285–299. doi: 10.1113/jphysiol.1978.sp012422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M. D., Bishop J. W., Cone C. H. Sensitization and habituation of the plantar cushion reflex in cats. Brain Res. 1976 Feb 20;103(2):215–228. doi: 10.1016/0006-8993(76)90795-2. [DOI] [PubMed] [Google Scholar]
- Egger M. D. Sensitization and habituation of dorsal horn cells in cats. J Physiol. 1978 Jun;279:153–166. doi: 10.1113/jphysiol.1978.sp012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M. D., Wall P. D. The plantar cushion reflex circuit: an oligosynaptic cutaneous reflex. J Physiol. 1971 Jul;216(2):483–501. doi: 10.1113/jphysiol.1971.sp009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Jami L., Laporte Y. Skeleto-fusimotor axons in the hind-limb muscles of the cat. J Physiol. 1975 Jul;249(1):153–166. doi: 10.1113/jphysiol.1975.sp011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Mannen H., Hongo T., Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979 Jul 15;186(2):189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Westman J. Intracellular application of horseradish peroxidase and its light and electron microscopical appearance in spinocervical tract cells. Brain Res. 1976 Apr 9;105(3):557–562. doi: 10.1016/0006-8993(76)90603-x. [DOI] [PubMed] [Google Scholar]
- Light A. R., Metz C. B. The morphology of the spinal cord efferent and afferent neurons contributing to the ventral roots of the cat. J Comp Neurol. 1978 Jun 1;179(3):501–515. doi: 10.1002/cne.901790304. [DOI] [PubMed] [Google Scholar]
- Proshansky E., Egger M. D. Dendritic spread of dorsal horn neurons in cats. Exp Brain Res. 1977 May 23;28(1-2):153–166. doi: 10.1007/BF00237093. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Rose P. K. Morphology of motoneurones in the upper cervical spinal cord of the adult cat [proceedings]. J Physiol. 1977 Oct;272(1):37P–38P. [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]