Fig. 1. The mouse prRDH gene, the targeting construct, and expression.
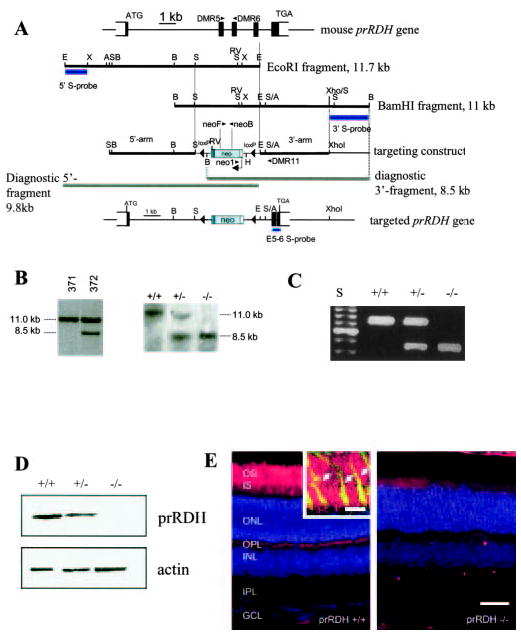
A, mouse prRDH gene structure, targeting construct, and targeted gene in which exons 2–4 have been deleted. The position of WT primers DMR5 and DMR6 in exons 2 and 3, respectively, is indicated. The 11.7-kb EcoRI fragment was used for cloning of the 5′-long arm, and the 11-kb BamHI fragment for cloning of the 3′-short arm. Bars underneath the genomic fragments indicate probes used for Southern blotting of ES cell line candidates. In the targeting construct, a fragment containing exons 2–4 was replaced by a neo cassette flanked by loxP sites. The extent of fragments representing the null allele is shown underneath the targeting construct. Bottom, schematic of the targeted prRDH gene. A, Acc65i; B, BamHI; E, EcoRI, H, HindIII; RV, EcoRV; S, SacI; X, XbaI; and XH, XhoI cleavage sites. In B: Left panel, Southern blot of BamHI-digested DNA of two ES cell lines hybridized with the 3′-probe; #371, negative cell line (no recombination); #372, positive clone showing the 11-kb WT allele and the 8.5-kb null allele. This line was used to generate the prRDH−/− mouse. Right panel, Southern blot with BamHI-digested DNA from SvJ WT, prRDH+/−, and prRDH−/− mice. The probe (E5–6 S-probe) was a 350-bp genomic fragment containing exon 5, intron 5, and exon 6 (see “Materials and Methods”). C, genotyping of prRDH+/+, prRDH+/−, and prRDH−/− mouse lines. The WT fragment was generated by PCR with DMR5 and DMR6 primers. The fragment corresponding to knock-out allele was generated by amplification with neo1 and DMR11 primers. Internal neo primers neoF and neoB (inside the coding sequence of neomycin phosphorylase) were used occasionally as an alternate primer pair. D, immunoblotting of proteins from the ROS extract from prRDH+/+ and prRDH−/− mice. The blot was developed using anti-prRDH polyclonal antibody generated against the C-terminal peptide derived from the prRDH sequence (“Materials and Methods”). The equal loading of the sample was verified by immunoblotting with anti-actin polyclonal antibody. E, immunocytochemical localization of prRDH (red) in mouse rod and cone outer segments. Eight-week-old frozen sections were probed with the polyclonal anti-prRDH antibody, generated as described under “Materials and Methods.” The specific response is present in the photoreceptors in the eye of prRDH+/+ mice and not in prRDH−/− mice. Scale bar, 50 μm. The nuclear layers were stained with Hoechst 33342 (Molecular Probes, Eugene, OR) dye. Inset, higher magnification of the photoreceptor outer segment layer. Anti-prRDH (red) is immunoreactive to both rod and cones (arrows). Cone sheaths (green) are labeled by fluorescein labeled PNA (peanut agglutinin). Yellow indicates colocalization. Scale bar, 10 μm.
