Abstract
An oxygen-assisted hydrocarbon chemical vapor deposition method is developed to afford large-scale, highly reproducible, ultra-high-yield growth of vertical single-walled carbon nanotubes (V-SWNTs). It is revealed that reactive hydrogen species, inevitable in hydrocarbon-based growth, are damaging to the formation of sp2-like SWNTs in a diameter-dependent manner. The addition of oxygen scavenges H species and provides a powerful control over the C/H ratio to favor SWNT growth. The revelation of the roles played by hydrogen and oxygen leads to a unified and universal optimum-growth condition for SWNTs. Further, a versatile method is developed to form V-SWNT films on any substrate, lifting a major substrate-type limitation for aligned SWNTs.
Keywords: nanomaterials, hydrocarbon chemistry, plasma, sp2–sp3 carbon, catalysis
Carbon nanotubes in aligned forms (1–6) are interesting for various scientific and practical applications ranging from electronics to biological devices. Although several chemical vapor deposition (CVD) methods have been developed to grow vertically aligned multiwalled carbon nanotubes, growth of vertical single-walled carbon nanotubes (V-SWNTs) is still in an early stage (7, 8). The formation of nanotube vertical films is indicative of high-yield growth from densely packed catalytic particles. That is, almost every particle produces a nanotube, and bundling of neighboring tubes leads to collective vertical growth. A key element in a recent ethylene CVD method that has obtained V-SWNT growth is the addition of ≈150 ppm water vapor (8). Another method that has obtained V-SWNT growth is an alcohol CVD method using ethanol as the carbon feedstock. In both cases, the key role played by the oxygen-containing species is suggested to be oxidation of amorphous carbon to facilitate SWNT growth.
Although we have made an effort to use both the water-assisted and alcohol CVD growth methods, we have been unable to obtain vertically aligned SWNTs, which indicates the narrow parameter space and growth window of these methods. Also, the specific roles played by the oxygen-containing species remain speculative and require further investigation. A more detailed understanding could lead to improved and more reproducible growth conditions. Here, we present a molecular oxygen-assisted plasma-enhanced CVD (PECVD) growth of V-SWNTs at the full 4-inch wafer scale. We find that adding oxygen (≈1%) to methane in the PECVD affords highly reproducible growth of densely packed SWNTs. Control experiments and knowledge from previous diamond PECVD work suggest that the key role played by oxygen in the high-yield SWNT growth is to balance C and H radicals and, specifically, to provide a C-rich and H-deficient condition to favor the formation of sp2-like graphitic SWNT structures. We reveal that reactive hydrogen species are generally unfavorable to SWNT formation and growth and can etch preformed SWNTs. The negative role of hydrogen and the positive role of oxygen have important implications to SWNT growth in general by various methods. Lastly, we present a simple but powerful method to form V-SWNT films on any desirable substrate (including metals and plastics) with strong interfacial adhesion.
Methods
Our nanotube synthesis was carried out in a conventional 4-inch thermal CVD system with an inductively coupled radio-frequency (RF) (13.56 MHz) plasma source located near the entrance of the growth gases (see the supporting information, which is published on the PNAS web site) (9). The substrates used were SiO2/Si with nominally 1- to 2-Å-thick Fe films (by quartz crystal thickness monitoring) deposited by electron beam evaporation. The thin Fe film was first annealed in oxygen at 550°C for 10 min and then heated in hydrogen to the growth temperature of 720°C. This treatment produced nearly a monolayer of Fe clusters on SiO2 with an average diameter of ≈1.3 nm as estimated from atomic force microscopy (AFM) measurements. We found that the formation of dense and relatively uniform particles was essential for V-SWNT growth. During nanotube growth, the compositions of gases in the tube furnace were methane (≈66%), hydrogen (≈12%), oxygen (≈1%), and Ar (≈21% as carrier gas), with a total pressure of 0.3–0.4 torr (1 torr = 133 Pa). The gas flow rates were CH4/H2/O2 = 160 sccm (standard cubic cm/min)/30 sccm/2.4 sccm. Ar was used as carrier gas for CH4 and O2. The percentage of partial pressures of various gases followed CH4:H2:O2 = 66%:12%:1% (the rest is Ar). The RF plasma was generated at a power of 60–70 W for 10–30 min for nanotube growth. This condition was highly reproducible in growing vertical SWNTs from run to run and from day to day.
Results and Discussion
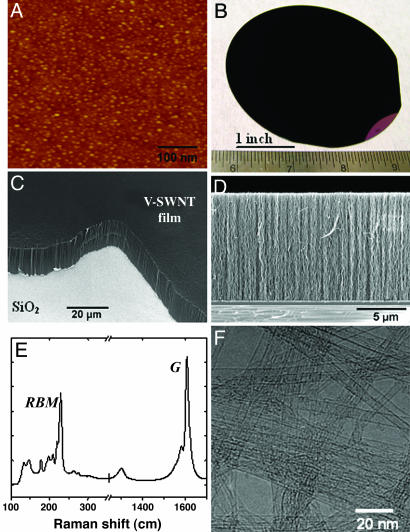
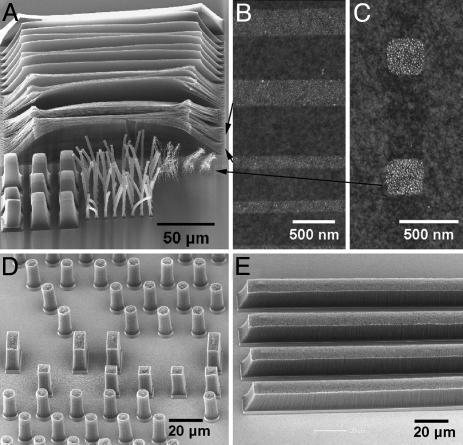
Our method produced nearly a monolayer of Fe clusters on 4-inch wafer scale SiO2 (Fig. 1A) substrates with an average diameter of ≈1.3 nm as estimated from AFM measurements. We found that the formation of dense and relatively uniform particles was essential for V-SWNT growth. With the submonolayer catalyst seeds and ≈1% oxygen added to our CVD, uniformly black nanotube films were grown on full 4-inch wafers (Fig. 1B). Scanning EM (SEM) revealed that the films consisted of nanotube strands oriented vertically to the substrate (Fig. 1 C and D). The length of the nanotubes is ≈10 μm for 10 min of growth and scaled approximately with growth time. Transmission EM (TEM) revealed exclusively SWNTs without any multiwalled carbon nanotubes or double-walled tubes (Fig. 1F). The TEM grids were prepared by sonicating a V-SWNT-covered substrate in a solvent and then drop-drying the suspension on the grid. Raman spectroscopy (Fig. 1E) of the as-grown samples identified resonant radial breathing modes of nanotubes in the range of 132–230 cm–1, corresponding to nanotubes 1–2 nm in diameter (10). The clear separation between the G peaks at ≈1,580 and ≈1,610 cm–1 (Fig. 1E) is also characteristic of SWNTs (10). By forming densely packed catalytic seed particles in lithographically defined regions shaped in squares, circles, or strips, we grew V-SWNTs to form square towers, circular towers, or sheets replicating the shape of the catalytic regions (Fig. 2) with the thickness of the sheets down to ≈100 nm (Fig. 2 A).
Fig. 1.
Molecular O2-assisted synthesis of high-yield V-SWNTs. (A) AFM image of a submonolayer of Fe nanoparticles (1–2 nm in topographic height) formed on a SiO2/Si wafer used for the synthesis. (B) Optical image of a visually black vertical SWNT film grown on a full 4-inch wafer. The tube-free region at the lower right of the wafer was due to clamping during deposition of Fe film. (C) A SEM image showing the slanted view of a V-SWNT film grown on SiO2/Si. (D) A SEM side view of a V-SWNT film. (E) Raman spectrum of a SWNT film. Laser excitation wavelength is 785 nm. (F) TEM image of SWNTs after they were sonicated off of the SiO2 and then dispersed onto a TEM grid. Note that using excessive O2 (>≈2%, below the ignition point of 4%) gives zero yield of SWNTs due to oxidation of nanotubes and should be avoided.
Fig. 2.
Molecular O2-assisted growth of vertical SWNT towers and sheets with submicrometer dimensions. (A) A SEM image showing SWNT towers with various widths (20 μm, 5 μm, 1 μm, 500 nm, and 300 nm from left to right in the lower region of the image) and vertical SWNT sheets (20 μm, 5 μm, 1 μm, 500 nm, 300 nm, and 100 nm thick from top to bottom of the upper part of the image) after 30 min of growth. (B) An AFM image of the patterned catalyst strips (bright 300-nm and 100-nm-wide regions) composed of densely packed Fe nanoparticles used for the growth of the 300-nm and 100-nm-thick vertical SWNT sheets (pointed to by arrows) in A. (C) An AFM image of two of the patterned catalyst squares (300 nm in width) used for the growth of the smallest towers (pointed to by an arrow and tilted due to high aspect ratio) in A. (D and E) Two SEM images of square and circular towers and lines of V-SWNTs, each from different growths than the sample in A, to show the reproducibility of the synthesis.
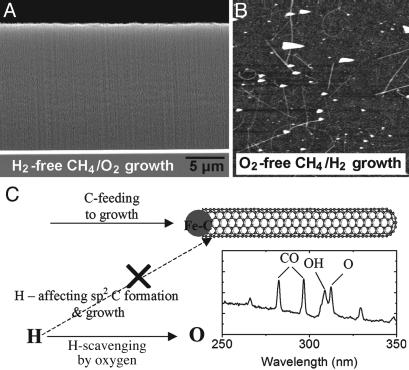
Our V-SWNT synthesis results are highly reproducible, and the optimum O2 concentration of ≈1% can be easily controlled experimentally. As mentioned earlier, the vertical orientation of SWNTs is indicative of high-yield growth with close to one-to-one growth of nanotubes from the seed particles. Through control experiments (Fig. 3 A and B) with varying concentrations of gases at a fixed temperature and total pressure, several growth trends emerged and shed light on the role played by oxygen in high-yield nanotube formation. Without the addition of O2, the same dense-catalyst monolayer failed to produce high yield of SWNTs packing into vertical films under any conditions tested. Without the H2 flow, however, vertical SWNTs can still be grown using CH4/O2 (Fig. 3A). Growth in CH4/H2 gave extremely low yield of SWNTs (Fig. 3B). These results indicated that H-rich conditions do not favor SWNT growth.
Fig. 3.
Roles of hydrogen and oxygen. (A) SEM of vertical SWNTs grown with CH4/O2 (partial pressure of O2 = 0.8%). (B) Very low yield of SWNTs grown in CH4/H2 (partial pressure of H2 = 7.4%). These control experiments were performed on the same catalyst/substrate and total pressure. (C) A schematic illustration of the proposed role of oxygen species in hydrocarbon-based synthesis of SWNTs. Scavenging of reactive H species by oxygen shuts off (shown by the “X”) the negative H effect to SWNT growth. We suggest that this desirable oxygen effect happens at the catalyst particle site where oxygen blocks H radicals from reacting with sp2 C networks formed on the metal seed particle. (Right) Optical emission spectrum recorded under our optimum V-SWNT growth condition with CH4/H2/O2 showing clear OH emission at 308.9 nm. The measurement was carried out by using a S2000 miniature fiber-optic spectrometer (Ocean Optics, Dunedin, FL). UV-grade quartz fiber was used to guide the plasma emission to the spectrometer.
We suggest that an important role played by oxygen in enhancing SWNT growth is the removal of reactive H radicals (relative to C species) that exist in hydrocarbon-based growth of nanotubes and that high concentrations of H species do not favor the formation and growth of sp2-like SWNTs (11) (Fig. 3). We first note that the CH4/H2/O2 gases have been used previously for sp3 diamond synthesis, albeit under H2-rich (>90%) and low CH4 (several percent) conditions (11, 12). Optical emission spectroscopy (Fig. 3C Right) has established that adding oxygen to the CH4/H2 plasma removes the highly reactive H radicals by means of H + O2 → OH + O with a large rate constant of ki0 ≈ 1017 cm3/mol·s (11). Oxygen can also remove and convert C species into CO by means of various reactions, but with lower rate constants (≈1014 cm3/mol·s) (11). Addition of O2 to CH4/H2 CVD can therefore provide an effective route to tune the ratio between C· and H· species. It has been found that the C·/H· ratio needs to be below a limit (H·-rich) for the formation of sp3 structure and that high C·/H· ratios favor sp2 carbon formation. The sp2 and sp3 carbon formation regimes can be controlled by changing oxygen concentration (11). These results and our SWNT growth results under varying H2 and O2 conditions indicate that high-yield synthesis with CH4/H2/O2 or CH4/O2 is due to oxygen removing H·, which removes or greatly reduces the negative effect of H species on SWNT growth (Fig. 3C).
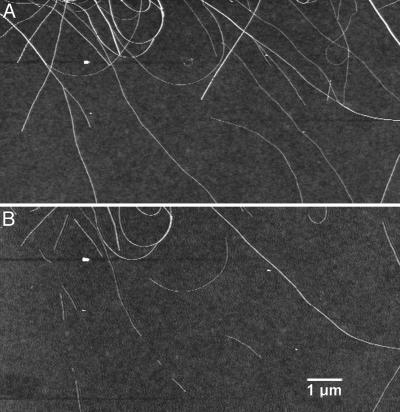
Our results lead to the conclusion that a favorable synthesis condition for SWNTs should employ sufficient C feedstock with few or no reactive H species, which may be a dilemma for hydrocarbon-based synthesis of SWNTs because H· is an inevitable product of hydrocarbon decomposition. This dilemma can potentially be addressed with controlled addition of oxygen to scavenge H·, enhancing the C·/H· ratio and thus favoring sp2 carbon production. High concentrations of reactive H· species are unfavorable to SWNT formation and growth, likely due to attacking of the sp2 CbyH· to form sp3 structures, (13, 14) giving low-yield growth of SWNTs (Fig. 3). Notably, hydrogenation and etching of SWNTs by H radicals generated in a plasma is known to occur even at room temperature (13). To confirm hydrogen attacking of SWNTs, we carried out control experiments and observed etching of preformed SWNTs by hydrogen plasma (Fig. 4) under various conditions ranging from room temperature to our typical growth temperature. This result provided direct evidence of the negative effect of reactive H species to the structures of SWNTs.
Fig. 4.
Hydrogen attacking of SWNTs. AFM images of nanotubes on a substrate recorded before (A) and after (B) H2 plasma treatment (5% in argon, total pressure = 0.5 torr, radio-frequency power = 20 W) at 500°C for 10 min. The treatment was carried out in the same chamber used for growth of the V-SWNTs with only the H2/Ar gas flow. The after-etching image in B clearly shows that some of the nanotubes in A were etched by H plasma. SWNTs are also found to be etched by H plasma at room temperature (not shown).
It is tempting to suggest that oxidizers play a cleansing role by etching amorphous carbon and thus maintaining high catalyst activity for SWNT growth, as suggested by the water- and alcohol-assisted growth work (7, 8). This cleansing role is not the key effect, at least in our case, because we find that amorphous carbon deposition can be avoided by using suitable CH4 concentrations by means of Ar dilution. Without oxygen, the yield of SWNTs (with any appreciable length) is low, suggesting the importance of oxygen in the initial nanotube nucleation and formation stage and not just during the sustained growth stage. Increased H2 presence always leads to systematic decrease in SWNT yield, with or without oxygen presence (see the supporting information). In our case, because of plasma-assisted decomposition, any H2 leads to very high H· concentrations, much more so than in thermal CVD. Thus, the observed extremely low yield of SWNTs for CH4/H2 growth (H2 < 10%) strongly suggests the negative blocking effect of H species to SWNT growth. With this negative H effect identified, we conclude that the favorable enhancement effect of oxygen occurs by means of removal of H species (Fig. 3 A vs. B). Indeed, our optical emission spectroscopic measurement under our optimum CH4/H2/O2 PECVD clearly identified significant OH species (Fig. 3C Right), lending direct spectroscopic evidence of the reaction of oxygen with H species in our SWNT growth process.
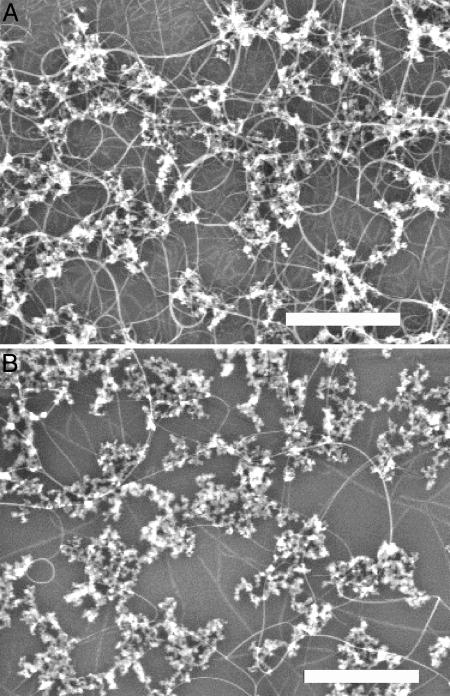
H scavenging by oxygen species could also be a factor in the high-yield SWNT growth by other methods (7, 8), although the precursors of oxidizing species differ. We carried out a control experiment to elucidate the effect of hydrogen to the growth yield of SWNT in the alcohol CVD growth process. We observed that increasing the H2 concentration while keeping the alcohol vapor pressure constant systematically reduces the yield of nanotubes (Fig. 5), providing evidence that hydrogen-rich environments are also undesirable and have negative effects on the yield of SWNTs in standard thermal CVD. Further, a connection can be made with non-hydrocarbon-based SWNT synthesis methods such as carbon monoxide CVD (15, 16). CO-CVD without the involvement of hydrogen indeed produces high yield of SWNTs, especially under high temperature and pressure when sufficient C feedstock is obtained (15). Other H-free high-yield growth of SWNTs includes laser ablation and arc discharge that vaporizes solid carbon without involving any hydrogen.
Fig. 5.
Effect of hydrogen in regular thermal CVD growth. SEM images of nanotubes grown from silica-supported Fe/Co/Mo catalysts deposited on substrates by regular thermal CVD using ethanol as the carbon feedstock in the presence of a H2 concentration of 1.9% (A) and 9.6% (B). Forming gas (300 sccm, 3% H2 in argon) was bubbled through EtOH held at –9°C in both growths. In addition to the bubbling forming gas, A had an additional 170 sccm of dry argon flow, and B had 35 sccm of dry argon and 135 sccm of dry H2 flows. The particles seen in the images are silica with supported catalytic metal species. The catalyst is made of Fe:Co:Mo (molar ratio of 1:1:0.2) acetate salts dissolved in anhydrous ethanol and sonicated with silica (0.72 mmol of metal/1 g of silica). The silica catalyst was deposited on a large silicon piece by means of spin-coating. After spin-coating, the chip was cut into two pieces, and one was used for A and the other for B. The yield of nanotubes is significantly higher in A than in B; surface “sheet” resistance for A and B is 25KΩ and 1.2MΩ, respectively. (Scale bars, 500 nm.)
A major difference between PECVD and thermal CVD is the much higher concentration of reactive radicals in the former. Small variations in the concentrations of molecules in PECVD can strongly affect the outcome of SWNT growth, as shown here (Fig. 3). For thermal CVD, the effect of varying the hydrogen concentration is much less pronounced than in the PECVD case, but it is still noticeable. Two advantages of PECVD over thermal CVD are the efficient decomposition of gas molecules and the fact that the concentration of reactive species can be sensitively tuned by the precursor concentration. Previously, many researchers have carried out PECVD synthesis of carbon nanostructures but have not succeeded in producing high-yield SWNTs. We suggest that an origin of the growth failure is the unobvious negative role played by reactive H species abundant in hydrocarbon PECVD. We believe that by adding suitable amounts of oxygen to the various types of PECVD systems, and by using dense and relatively uniform catalyst particles, one should be able to readily produce SWNTs at high yields. Oxygen-assisted PECVD could thus become a powerful and widely used method for efficient production of SWNTs. Note that we have attempted adding O2 (0.04–4% partial pressure) to regular thermal CVD of hydrocarbons (17, 18) without achieving successful growth of high-yield V-SWNTs. This phenomenon is not understood currently, and fine-tuning of O2 concentration might be needed to enhance SWNT growth in thermal CVD processes.
We also note an effect of H species on the diameter distribution of SWNTs synthesized by CVD. The blocking of SWNT formation by reactive H species appears to be more pronounced for smaller diameter tubes. Theoretically, smaller SWNTs are more susceptible to hydrogenation by H species because of higher tube curvature and a higher sp3 formation tendency (14). In our H plasma etching experiments, we observed the trend that smaller SWNTs tended to be attacked preferentially over larger ones, as seen in the AFM images in Fig. 4. This finding is also consistent with the observation that oxygen-free hydrocarbon CVD (17, 18) generally produces large (2–3 nm) SWNTs with few tubes ≤1 nm (when particles of various sizes <≈4–5 nm exist). In stark contrast, without hydrogen, CO-based CVD methods frequently produce abundant SWNTs in the 0.7- to 1.5-nm range. Our O2-assisted CH4 PECVD presented here also synthesizes abundant SWNTs in the 1-nm range (see Raman data in Fig. 1E). The growth of vertical multiwalled carbon nanotubes should be less affected by H blocking due to the higher stability of larger tubes (14) and has been readily achieved by hydrocarbon CVD without any oxygen assistance (2–6).
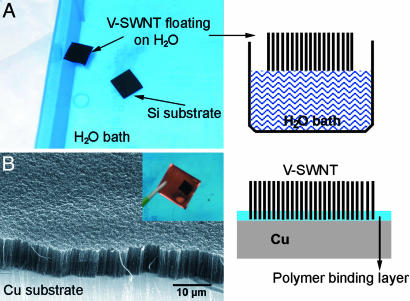
Lastly, we present a unique method for obtaining vertically aligned SWNTs on a wide range of substrates, including metals and polymers. This goal has been elusive thus far because of the incompatibility of many types of substrates with the high growth temperature of SWNTs, but it could be the key to the utility of aligned SWNT materials. Our method is simple and involves “lifting off” the V-SWNT by using HF (1% for 10 s) to etch the underlying SiO2 layer and subsequently free-floating the V-SWNT film on a water surface (Fig. 6A). After liftoff, the V-SWNTs can be transferred to other substrates coated with an interfacial thin polymer [(50-nm polymethylmathacrylate (PMMA)] layer for adhesion (Fig. 6B). After transfer, the substrate is heated to >190°C, well above the glass transition temperature of PMMA (≈105°C) for melting of the polymer layer and “gluing” the substrate to the ends of the SWNTs in the vertical film. This gluing effect affords strongly adhering vertical SWNTs on various substrates, including Cu (Fig. 6B Inset), polymers, and glasses. The V-SWNT films thus derived are robust and do not lift off from substrates, even after immersion in ethanol or acetone solvents. This development may greatly expand the utility of V-SWNTs. For instance, it has been suggested that vertically aligned SWNT films can be used as a thermal interface material for heat conduction and dissipation of microelectronics chips. A low-temperature process is needed to form V-SWNTs on these chips without harming the preformed circuitry. Our room temperature SWNT transfer approach meets this challenge.
Fig. 6.
How to form vertical SWNT films on a wide range of substrates. (A) Photograph of a V-SWNT film free-floating on water after being lifted off from a SiO2/Si substrate by HF etching of the SiO2 layer underlying the SWNTs. (Right) A schematic drawing of the free-floating SWNT film with nanotubes held together by van der Waals interactions. (B) SEM image of a vertical SWNT film after transferring onto a copper substrate with a thin polymer-binding layer at the Cu–SWNT interface. (Inset) A photograph showing a vertical SWNT film (black) on Cu. (Right) A schematic drawing depicting the vertical nanotube film and the Cu interface.
Conclusions
We have presented a molecular oxygen-assisted PECVD growth of high yield of vertically aligned SWNTs at the full 4-inch wafer scale. Various control experiments and knowledge from previous diamond PECVD work revealed the negative effect of hydrogen species to the formation and growth of SWNTs as well as etching effects of hydrogen plasma to preformed SWNTs. The key role played by oxygen in our high-yield SWNT growth is to balance C and H radicals and, specifically, to provide a C-rich and H-deficient condition to favor the formation of sp2-like graphitic structures. This understanding has important implications for SWNT growth in general by various methods. With the addition of suitable amounts of oxygen to suppress H species, we expect that various types of PECVD setups can produce SWNTs at ultra-high yield and efficiency. Further, we present a method to form V-SWNT films on any desirable substrate (including metals and plastics) with strong interfacial adhesion.
Supplementary Material
Acknowledgments
This work was supported in part by the Stanford University Global Climate and Energy Project.
Author contributions: H.D. designed research; G.Z., D.M., L.Z., A.J., Y.L., E.Y., Q.W., and J.P.M. performed research; Y.N. and J.G. contributed new reagents/analytic tools; G.Z., D.M., and H.D. analyzed data; and H.D. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: SWNT, single-walled carbon nanotube; V-SWNT, vertical SWNT; CVD, chemical vapor deposition; PECVD, plasma-enhanced CVD; AFM, atomic force microscopy; sccm, standard cubic cm/min; SEM, scanning EM; TEM, transmission EM.
References
- 1.Li, W., Xie, S., Qian, L. & Chang, B. (1996) Science 274, 1701–1703. [DOI] [PubMed] [Google Scholar]
- 2.Ren, Z. F., Huang, Z. P., Xu, J. W., Wang, J. H., Bush, P., Siegal, M. P. & Provencio, P. N. (1998) Science 282, 1105–1107. [DOI] [PubMed] [Google Scholar]
- 3.Fan, S., Chapline, M., Franklin, N., Tombler, T., Cassell, A. & Dai, H. (1999) Science 283, 512–514. [DOI] [PubMed] [Google Scholar]
- 4.Jiang, K. L., Li, Q. Q. & Fan, S. S. (2002) Nature 419, 801. [DOI] [PubMed] [Google Scholar]
- 5.Dai, H. (2002) Surf. Sci. 500, 218–241. [Google Scholar]
- 6.Melechko, A. V., Merkulov, V. I., McKnight, T. E., Guillorn, M. A., Klein, K. L., Lowndes, D. H. & Simpson, M. L. (2005) J. Appl. Phys. 97, 041301. [DOI] [PubMed] [Google Scholar]
- 7.Murakami, Y., Chiashi, S., Miyauchi, Y., Hu, M. H., Ogura, M., Okubo, T. & Maruyama, S. (2004) Chem. Phys. Lett. 385, 298–303. [Google Scholar]
- 8.Hata, K., Futaba, D. N., Mizuno, K., Namai, T., Yumura, M. & Iijima, S. (2004) Science 306, 1362–1364. [DOI] [PubMed] [Google Scholar]
- 9.Li, Y. M., Mann, D., Rolandi, M., Kim, W., Ural, A., Hung, S., Javey, A., Cao, J., Wang, D. W., Yenilmez, E., et al. (2004) Nano Lett. 4, 317–321. [Google Scholar]
- 10.Jorio, A., Saito, R., Hertel, T., Weisman, R. B., Dresselhaus, G. & Dresselhaus, M. S. (2004) MRS Bull., 276–280.
- 11.Benndorf, C., Joeris, P. & Kroger, R. (1994) Pure Appl. Chem. 66, 1195–1206. [Google Scholar]
- 12.Eaton, S. & Sunkara, M. K. (2000) Diamond Related Mater. 9, 1320–1326. [Google Scholar]
- 13.Nikitin, A., Ogasawara, H., Mann, D., Zhang, Z., Dai, H., Cho, K. J. & Nilsson, A. (2005) Phys. Rev. Lett., cond-mat/0510399. [DOI] [PubMed]
- 14.Park, S., Srivastava, D. & Cho, K. (2003) Nano Lett. 3, 1273–1277. [Google Scholar]
- 15.Nikolaev, P., Bronikowski, M. J., Bradley, R. K., Rohmund, F., Colbert, D. T., Smith, K. A. & Smalley, R. E. (1999) Chem. Phys. Lett. 313, 91–97. [Google Scholar]
- 16.Kitiyanan, B., Alvarez, W. E., Harwell, J. H. & Resasco, D. E. (2000) Chem. Phys. Lett. 317, 497–503. [Google Scholar]
- 17.Kong, J., Soh, H., Cassell, A., Quate, C. F. & Dai, H. (1998) Nature 395, 878. [Google Scholar]
- 18.Hafner, J., Bronikowski, M., Azamian, B., Nikolaev, P., Colbert, D. & Smalley, R. (1998) Chem. Phys. Lett. 296, 195–202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.