Fig. 3.
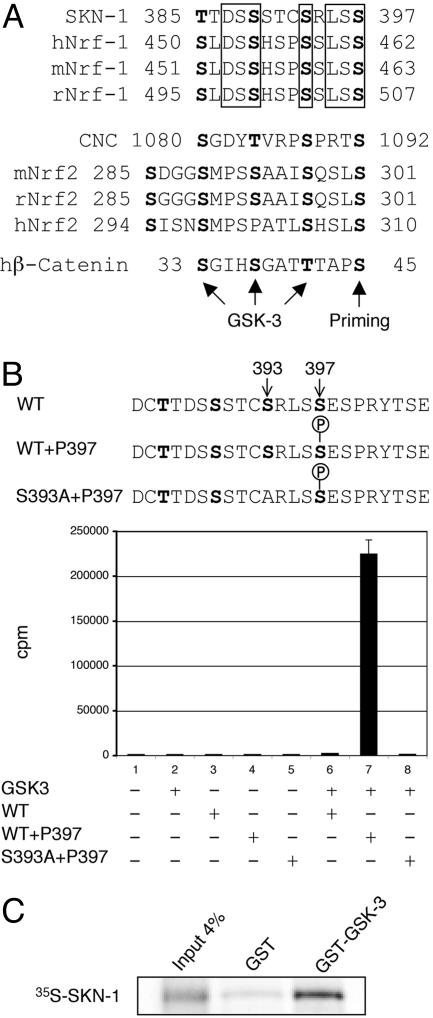
Phosphorylation of SKN-1 by GSK-3. (A) A GSK-3 phosphorylation motif in C. elegans SKN-1 is conserved in mammalian Nrf1 proteins. This motif, which has three predicted GSK-3 sites, is similar in structure to the GSK-3 target motif in β-catenin, in which priming phosphorylation at Ser-45 by casein kinase-1α is followed by sequential GSK-3 phosphorylation at Thr-41, Ser-37, and Ser-33 (26). Predicted compound GSK-3 phosphorylation motifs are also present in mammalian Nrf2 and the orthologous Drosophila protein CNC. Conserved residues in each group are boxed. h, human; m, mouse; r, rat. (B) Phosphorylation of SKN-1 Ser-393 by GSK-3β in vitro. GSK-3β phosphorylates the SKN-1 peptide shown provided that phosphorylated serine is present at position 397. This phosphorylation depends on the presence of Ser-393. cpm incorporated in a representative phosphorylation assay are graphed. (C) SKN-1 binds specifically to a C. elegans GST–GSK-3 fusion protein in vitro.