Abstract
Thyroid hormone (T3) and peroxisome proliferators have overlapping metabolic effects in the maintenance of lipid homeostasis. Their actions are mediated by their respective receptors: thyroid hormone receptors (TR) and peroxisome proliferator-activated receptors (PPAR). We recently found that a dominantly negative TRβ mutant (PV) that causes a genetic disease, resistance to thyroid hormone, acts to repress the ligand (troglitazone)-mediated transcriptional activity of PPARγ in cultured thyroid cells. This finding suggests that TRβ mutants could crosstalk with PPARγ-signaling pathways. The present study explored the molecular mechanisms by which PV represses the PPARγ transcriptional activity. Gel-shift assays show that the PV, similar to wild-type TRβ, bound to the peroxisome proliferator response element (PPRE) as homodimers and heterodimers with PPARγ or the retinoid X receptor (RXR), thereby competing with PPARγ for binding to PPRE and for sequestering RXR. Association of PPRE-bound PV with corepressors [e.g., nuclear receptor corepressor (NCoR)] that led to transcriptional repression was independent of T3 and troglitazone. Chromatin immunoprecipitation assay further demonstrated that, despite the presence of ligands, NCoR was recruited to PPRE-bound PV on a PPARγ-target gene, the lipoprotein lipase, in vivo, suggesting the dominant action of PV on PPARγ-mediated transcriptional activity. Thus, the dominant negative action of PV is not limited on the wild-type TRs. The findings that TRβ mutants affect PPARγ functions through dominant negative action provide insights into the molecular mechanisms by which TR regulates the PPARγ-target genes involved in metabolic pathways, lipid homeostasis, and carcinogenesis.
Keywords: chromatin immunoprecipitation, dominant negative activity, thyroid hormone receptor mutant, transcription regulation
Thyroid hormone receptors (TRs) are ligand-dependent transcription factors that regulate growth, differentiation, and maintenance of metabolic homeostasis. TRs are encoded by α and β genes, located on chromosomes 3 and 17, respectively. Alternative splicing of the primary transcripts gives rise to four thyroid hormone (T3)-binding proteins, α1, β1, β2, and β3, which bind to thyroid hormone response elements (TRE) on the promoters of T3-target genes. The consensus TREs consist of two half-sites with the sequence of (A/G)GGT(C/G/A)A that are arranged as a direct repeat, separated by four nucleotides (DR4), palindrome (Pal), or inverted repeat, separated by six nucleotides (F2) (1). The transcriptional activity of TRs is modulated by a host of coregulatory proteins (2). In the absence of T3, TRs repress basal transcription through association with a variety of corepressors, such as NCoR and the silencing mediator for retinoid and thyroid hormone receptors. Binding of T3 induces structural changes to release the corepressors and to allow recruitment of coactivators, such as the steroid hormone receptor coactivator-1 (SRC-1) and other p160 family members. Corepressors harbor deacetylase activity that acts to modify the chromatin structure so as to limit the access of basal transcriptional machinery. Coactivator complexes, in contrast, harbor histone acetyltransferase and methyltransferase activities that facilitate transcription by rendering chromatin more accessible to transcription factors (1, 2).
We have created a mutant mouse by targeting a mutation (PV) to the TRβ gene locus (TRβPV mice) (3). PV was identified in a patient (PV) with resistance to thyroid hormone (RTH) (4). RTH is caused by mutations of the TRβ gene and manifests symptoms due to decreased sensitivity to T3 in target tissues (5). The most common form of RTH is familial, with autosomal-dominant inheritance (5). Patients are usually heterozygotes, with only one mutant TRβ gene, and the symptoms are mild. Moreover, clinical manifestations are variable among families with RTH and also among affected family members. Clinical features include goiter, short stature, decreased weight, hypercholesterolemia, tachycardia, hearing loss, attention deficit-hyperactivity disorder, decreased IQ, and dyslexia (5). One single patient homozygous for a mutant TRβ with a complex phenotype of extreme RTH has been reported (6). PV has a C-insertion at codon 448 that produces a frame-shift in the carboxyl-terminal 14 amino acids of TRβ1 (4). PV has completely lost T3 binding and exhibits potent dominant negative activity (7). Patient PV has only one mutated TRβ allele and exhibits short stature and delayed bone age, similar to other heterozygous RTH patients (5).
Remarkably, as homozygous TRβPV mice (TRβPV/PV mice) age, they spontaneously develop follicular thyroid carcinoma through a pathological progression resembling human thyroid cancer (8). Gene-expression profilings of thyroid tumors in TRβPV/PV mice have identified the repression of the peroxisome proliferator-activated receptor γ (PPARγ)-signaling pathway as one of the altered pathways that contribute to thyroid carcinogenesis (9). These findings suggest that TRβ mutants could cross talk with the PPARγ-signaling pathway.
PPARs are also members of the nuclear hormone-receptor superfamily and play an important role in adipogenesis, cell cycle control, apoptosis, and carcinogenesis. PPARs are activated by a broad class of structurally diverse xenobiotic chemicals termed peroxisome proliferators (10, 11). PPARs bind to peroxisome proliferator response elements, a direct repeat of the AGG(T/A)CA binding motif, separated by one nucleotide (DR1). Similar to TRs, PPARs require heterodimerization with retinoid X receptors (RXRs) for optimal binding to DNA to activate peroxisome proliferator-target genes (12). Cell-based studies indicate that PPARs are able to selectively inhibit the transcriptional activity of TRs by competing for RXR (13, 14). Conversely, TRα1 was shown to regulate the expression of acyl-CoA oxidase peroxisome proliferator response element (PPRE)-mediated transcriptional activity (15), suggesting that these two receptors could regulate a similar subset of genes involved in maintaining lipid metabolism.
The observation that a TRβ mutant, PV, represses the PPARγ-signaling pathway in the thyroid of TRβPV/PV mice (9) prompted us to delineate its underlying molecular mechanisms. We found that, besides the liganded TRβ, the unliganded TRβ and the mutant PV also compete with PPARγ for binding to PPRE as homodimers and heterodimers with PPARγ or with RXR. Cell-based studies indicate that the unliganded TRβ and the mutant PV repress the troglitazone-dependent transcriptional activity of PPARγ. Chromatin immunoprecipitation (CHIP) assay demonstrates that the repression is due to the recruitment of corepressors to the promoter of PPARγ-target genes in vivo. Importantly, the recruitment of corepressors to the PPRE-bound PV on the promoter is T3- and troglitazone-independent. Thus, the present study shows that PV is a dominant negative regulator of PPARγ action.
Materials and Methods
Mouse Strain and Cell Culture. The mice harboring the TRβPV gene were prepared and genotyped as described in ref. 3. The animal protocol was approved by the National Cancer Institute Animal Care and Use Committee.
Transient Transfection. Transient transfection experiments were carried out in CV-1 cells similarly to the method described in Zhu et al. (16), except with the use of FuGENE 6 (Roche Molecular Biochemicals) according to the manufacturer's protocol. Cells (1.5 × 105 cells per well) were transfected with pPPRE-TK-Luc (0.4 μg) and PPARγ1 expression vector (pSG5/stop-mPPARγ, 0.2 μg) in the absence or increasing amounts of TRβ1 or PV expression vectors [pCLC51 (16) or pCLC51PV (17), respectively]. SRC-1 expression vector (pIRES-SRC-1, 1 μg) was cotransfected into cells in some experiments. Twenty-four hours after transfection, 20 μM troglitazone or 100 nM T3 was added and incubated for an additional 24 h before harvest. All experiments were performed in triplicate and repeated three times. The results shown are the mean ± SD.
EMSA. The double-stranded oligonucleotide containing the PPR E (PPR E-5′, GAACGTGACCTTTGTCCTGGTCCCCTTTGCT and PPRE-3′, GGGACCAGGACAAAGGTCACGTTCGGGAAAGG) (the underlined portion of the sequence is the PPRE for Acyl-CoA oxidase, a target gene for PPARγ) (18) was labeled with [32P]dCTP similarly to the method described by Ying et al. (19). Approximately 0.2 ng of probe (3-5 × 104 cpm) was incubated with in vitro-translated PPARγ, TRβ1, or PV, with or without RXRβ (2 μl) in the binding buffer. Anti-TRβ1 (C4) (20), anti-PV (T1) (20), and/or anti-PPARγ H-100 (Santa Cruz Biotechnology) were used in the supershift experiments.
Preparation of Nuclei and CHIP. Nuclei from thyroid tissues were isolated as described in refs. 21 and 22. The CHIP assay was performed by using a CHIP assay kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer's instructions. Chromatin solution (1 ml) was immunoprecipitated with 5 μl of anti-TRβ1 antibody, (C4; 20), anti-PV [T1 (20) or #302 (17)], anti-PPARγ (H-100, Santa Cruz Biotechnology), anti-NCoR antiserum (a generous gift of J. Wong, Baylor College of Medicine, Houston), or IgG as control. The recovered DNA was used as a template for amplification using quantitative real-time PCR. Two percent of the chromatin solution (20 μl) was used for the control of input DNA. The primer sequences for the lipoprotein lipase (LPL) PPRE are forward primer, 5′-CCTCCCGGTAGGCAAACTG-3′ and reverse primer, 5′-AACGGTGCCAGCGAGAAG-3′. The amplified DNA was analyzed on 2% agarose gel with ethidium bromide staining.
Results
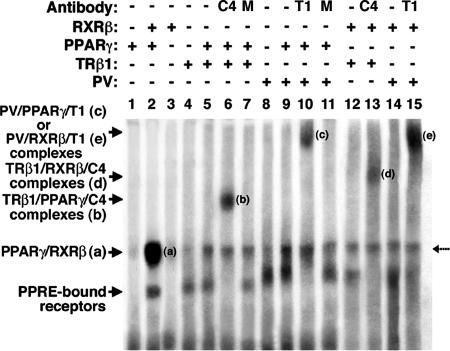
PV Binds to PPRE as Heterodimers with PPARγ or RXR. We have reported that PV represses troglitazone-dependent transcriptional activity of PPARγ in a cultured thyroid cell line, PC cells (19). However, it was not clear by what mechanisms PV acts to repress the troglitazone-dependent transcriptional activity of PPARγ. We hypothesized that the repression could be due to the competition of PV with PPARγ for binding to PPRE. Because it is unknown whether PV can bind to PPRE (DR1), we evaluated its binding to PPRE by EMSA. Fig. 1, lane 2 shows that PPARγ binds to PPRE strongly as heterodimers with RXRβ (band “a”), but binding to PPRE as homodimers was not detectable under the experimental conditions (Fig. 1, lane 1). No binding of RXR homodimers to PPRE was observed (Fig. 1, lane 3). However, binding of TRβ1 to PPRE as homodimers (Fig. 1, lane 4) or as heterodimers with PPARγ was detected (Fig. 1, lane 5). The latter was confirmed by using supershift experiments in which anti-TRβ1 antibody C4 (Fig. 1, lane 6) specifically shifted the PPRE-bound PPARγ/TRβ1 heterodimers to a more retarded position by EMSA (band “b”) but not by an irrelevant antibody (MOPC, Fig. 1 lane 7). PV also bound to PPRE as homodimers (Fig. 1, lane 8) or as heterodimers with PPARγ (Fig. 1, lane 9). The latter was confirmed by supershifting the PPRE-bound PPARγ/PV to a more retarded position with anti-PV antibody T1 (Fig. 1, lane 10, band “c”). However, an irrelevant antibody failed to do so (Fig. 1, lane 11). The use of anti-PPARγ antibody in the supershift experiments further confirms the binding of PPARγ/TRβ1 and PPARγ/PV heterodimers to PPRE (data not shown). Similar to the binding of TRs to TREs, the binding of TRβ1 or PV to PPRE as heterodimers was not affected by the ligand for TRβ1 (T3) or for PPARγ (troglitazone). The ligand-independent association of TRβ1 with PPARγ in cells was also demonstrated by coimmunoprecipitation experiments (data not shown).
Fig. 1.
Binding of PPARγ,TRβ1, or PV to PPRE by EMSA. Lysate containing in vitro-translated PPARγ,TRβ1, or PV proteins (5 μl) in the presence or absence of RXRβ or anti-TRβ1 (C4, 2 μg), PV (T1, 2 μg), or an irrelevant antibody, MOPC (M, 2 μg) antibodies were incubated with 32P-labeled PPRE and analyzed by gel retardation, as described in Materials and Methods. Amounts of lysate were kept constant by the addition of unprogrammed lysate, as needed. Lanes are as marked. The broken arrow denotes the nonspecific band.
TRβ1 or PV also bound to PPRE as heterodimers with RXRβ (Fig. 1, lanes 12 and 14, respectively). This binding was confirmed by supershifting the PPRE-bound TRβ1/RXRβ or PV/RXRβ with anti-TRβ1 C4 or anti-PV T1 to a more retarded position (Fig. 1, lane 13, band “d” and lane 15, band “e,” respectively). These results indicate that TRβ1 and the mutant PV can bind to PPRE as homodimers and as heterodimers with PPARγ or with RXRβ.
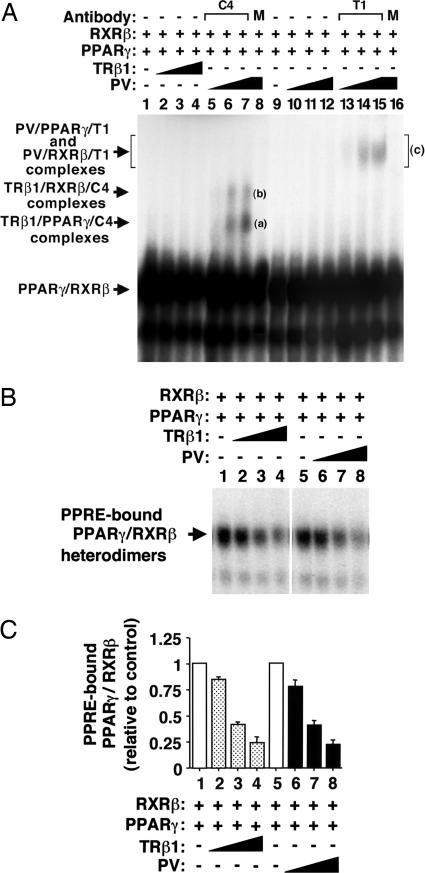
PV Inhibits the Binding of PPARγ with the RXR to Form PPRE-Bound Heterodimers in a Dose-Dependent Manner. Because both PPARγ and TRs heterodimerize with RXR on their cognate hormone-response elements, the finding that both TRβ1 and PV bound to PPRE as heterodimers with RXRβ or PPARγ prompted us to ask whether TRβ1 or PV could compete with PPARγ for binding to PPRE as heterodimers with RXR or PPARγ. Fig. 2 shows that, indeed, compared with PPRE-bound PPARγ/RXR in the absence of TRβ1 (Fig. 2 A, lane 1) or PV (Fig. 2 A, lane 9), binding of PPARγ/RXRβ to PPRE was decreased in the presence of increasing concentrations of TRβ1 (Fig. 2 A, lanes 2-4) or PV (Fig. 2 A, lanes 10-12). This concentration-dependent decrease in the binding can be seen more readily in the autoradiogram with a shorter exposure (Fig. 2B, lanes 2-4 for TRβ1 and lanes 6-8 for PV).
Fig. 2.
Inhibition of the PPARγ/RXR heterodimers binding to PPRE by increasing amounts of TRβ1 or PV. (A) Lysates containing PPARγ and RXRβ proteins (1 μl) were incubated in the absence or presence of TRβ1 (1, 5, or 10μl for lanes 2, 3, and 4 and 5, 6, and 7; PV (1, 5, or 10 μl for lanes 10-12 and 13-15) or M (MOPC, an irrelevant monoclonal antibody, lanes 8 and 16) with 32P-labeled PPRE and analyzed by EMSA, as described in Materials and Methods. Lanes are as marked. (B) Autoradiograph of the results from lanes 1-4 and 9-12 from A with shorter exposure time to illustrate the competition in the binding of PPARγ/RXR by either TRβ1 (lanes 2-4) or PV (lanes 6-8). (C) The intensities of bands in B were quantified by using the Astra 6450 scanner (UMAX Technologies, Dallas), and the data were analyzed by using the program nih image 1.61. The data are expressed as mean ± SD (n = 3).
To verify that TRβ1 or PV competed with PPARγ for binding to PPRE, anti-TRβ1 (C4) and anti-PV (T1) antibodies were used to confirm the formation of PPRE-bound TRβ1/RXRβ and TRβ1/PPARγ or PV/RXRβ and PV/PPARγ on PPRE by EMSA. As shown in Fig. 2 A, lanes 5-7, two more retarded supershifted bands (bands “a” and “b”) were detected. On the basis of supershifted bands shown in Fig. 1, lane 6 for TRβ1/PPARγ/C4 complexes and in Fig. 1, lane 13 for TRβ1/RXRβ/C4 complexes, the less-retarded band “a” in Fig. 2 A, lanes 5-7 represented TRβ1/PPARγ/C4 complexes, and the more-retarded band “b” represented PPRE-bound TRβ1/RXRβ/C4 complexes. The specificity of these supershifted bands was verified by using an irrelevant antibody MOPC, for which no supershifted band was noted in Fig. 2 A, lane 8. Because the PPRE-bound PV/PPARγ or PV/RXRβ were supershifted to a similarly retarded position by T1, as shown in Fig. 1, lanes 10 and 15, respectively, the more-retarded broad band c in Fig. 1, lanes 13-15 represented both PV/PPARγ/T1 and PV/RXRβ/T1 complexes. Again, the specificity of supershifted band c was confirmed by using an irrelevant antibody MOPC, for which no supershifted band was observed in Fig. 1, lane 16.
The intensities of the PPARγ/RXRβ heterodimer bands shown in Fig. 2B were quantified, and the results are graphed in Fig. 2C. In these experiments, equal amounts of TRβ1 or PV proteins were added at each pair of corresponding lanes (i.e., Fig. 2C, lanes 2 and 6, lanes 3 and 7, and lanes 4 and 8 for TRβ1 and PV, respectively). No significant differences were observed in the extent of reduction of PPRE-bound PPARγ/RXRβ by the presence of TRβ1 or PV proteins at the corresponding concentrations. These results suggest that PV and TRβ1 are similarly effective in competing with PPARγ for binding to PPRE as heterodimers with PPARγ or RXRβ.
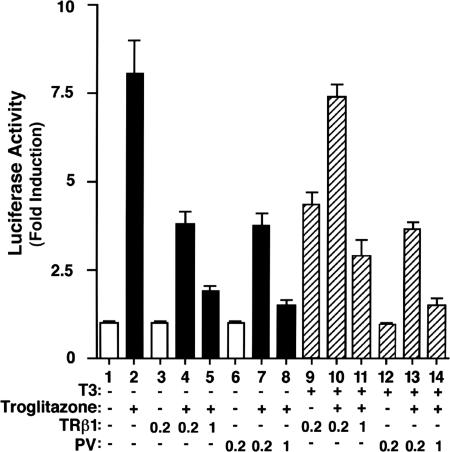
PV Represses the Ligand-Dependent Transactivation of PPARγ in CV-1 Cells. The above EMSA results suggest that the repression of troglitazone-dependent PPARγ-mediated transactivation by PV would not be limited to the mouse thyroid PC cells (19). We therefore examined whether this repression also occurs in monkey CV-1 cells. The luciferase-reporter-containing PPARγ response element (AGGTACXAGGTCA, DR1) and mouse PPARγ1 expression vector were cotransfected with or without TRβ1 or PV into cultured CV-1 cells in the presence or absence of troglitazone or T3 (Fig. 3). The transactivation activity of PPARγ was clearly stimulated by troglitazone (8.1-fold) (Fig. 3, compare bar 2 with bar 1). In the absence of T3, increasing the concentration of unliganded TRβ1 repressed the troglitazone-dependent transactivation activity of PPARγ (Fig. 3, bars 4 and 5, a 51% and 75% reduction at the TRβ1 expression plasmid concentrations of 0.2 and 1.0 μg, respectively). Similar repression of the troglitazone-dependent transactivation activity of PPARγ by PV was also detected (Fig. 3, bars 7 and 8). In the presence of T3, however, a different profile of transactivation activity emerged. At a low concentration of TRβ1 expression plasmid (0.2 μg), no repression of troglitazone-dependent transactivation activity of PPARγ was detected (Fig. 3, compare bar 10 with bar 2), but, at a higher concentration of TRβ1 (1.0 μg), repression of troglitazone-dependent transactivation activity of PPARγ was detected (60% reduction, Fig. 3, compare bar 11 with bar 2). PV, however, repressed the troglitazone-dependent transactivation activity of PPARγ in a dose-dependent manner (54% and 81% for 0.2 and 1.0 μg, respectively; Fig. 3, compare bars 13 and 14 with bar 2), similar to that in the absence of T3 (Fig. 3, bars 7 and 8). These results indicate that, in addition to PC cells, PV and the unliganded TRβ1 repressed the troglitazone-dependent transactivation activity of PPARγ in CV-1 cells.
Fig. 3.
PV represses the ligand-dependent transactivation of PPARγ in monkey CV-1 cells. CV-1 cells were cotransfected with 0.4 μg of the reporter plasmid (pPPRE-TK-Luc), 0.2 μg of pSG5/stop-mPPARγ1 for PPARγ1, and various cDNA expression vectors [empty vector, pCLC 51 for TRβ1 (0.2 μgor1 μg) and pCLC51PV for PV (0.2 μg or 1 μg)], as indicated. Cells were treated with either DMSO as vehicle or troglitazone (20 μM) in the absence or presence of T3 (100 nM), as marked. Data were normalized against the protein concentration in the lysates. Relative luciferase activity was calculated and shown as fold-induction relative to the luciferase activity of PPRE in the cells treated with DMSO in the absence of T3, defined as 1. The data are expressed as mean ± SD (n = 3).
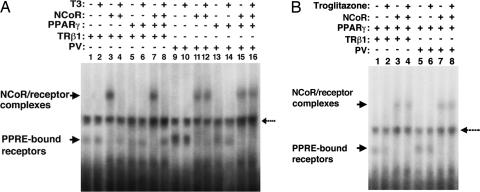
NCoR Constitutively Associates with PPRE-Bound PV Independent of Troglitazone or T3. It has been shown that TRβ mutants interact aberrantly with corepressors such as NCoR and exhibit an impaired ability to dissociate from corepressors in the presence of T3 (23). Importantly, a strong positive correlation was found between mutant-receptor interactions with corepressors and transcriptional silencing activity (24). That PV, similar to the unliganded TRβ1, repressed the trogliotazone-dependent transcriptional activity of PPARγ (Fig. 3) suggests that PV could constitutively associate with corepressors, such as NCoR, and thereby prevent the recruitment of coactivators to the promoters of PPARγ-target genes. Therefore, we used EMSA to assess ligand-independent association of PV with NCoR on PPRE (Fig. 4). For control, we first confirmed that PPRE-bound unliganded TRβ1 associated with NCoR, as indicated by the presence of more retarded unliganded TRβ1/NCoR complexes (Fig. 4, compare lane 3 with lane 1). Fig. 4, lanes 1 and 2 show that binding of TRβ1 to PPRE was T3-independent, similar to the binding of TRβ1 to TREs (1). Binding of T3 to TRβ1 released NCoR from the PPRE-bound TRβ1 (Fig. 4A, lane 4). In the presence of T3, no association of NCoR with either PPRE-bound TRβ1 homodimers or heterodimers with PPARγ was found (Fig. 4A, compare lane 8 with lane 7). Similar to the unliganded TRβ1, PV associated with NCoR in the absence of T3 as a homodimer (Fig. 4A, lane 11) or heterodimer (Fig. 4A, lane 15) with PPARγ. However, in contrast to the liganded TRβ1, the presence of T3 could not release NCoR from PPRE-bound PV homodimers (Fig. 4A, lane 12) or heterodimers (Fig. 4A, lane 16). These results indicate that PPRE-bound PV constitutively associates with NCoR.
Fig. 4.
Association of NCoR with PPRE-bound unliganded TRβ1 or PV is independent of troglitazone. In vitro-translated NCoR, PPARγ, TRβ1, or PV proteins (3.5 μl) in the presence or absence of 1 μMT3(A)or1 μM troglitazone (B) were incubated with 32P-labeled PPRE and analyzed by EMSA, as described in Materials and Methods. The broken arrow denotes the nonspecific binding.
Whether the PPARγ ligand troglitazone affected the association of NCoR with PPRE-bound TRβ1/PPARγ or PV/PPARγ heterodimers was also examined. Fig. 4B shows that troglitazone did not affect the binding of TRβ1 to PPRE as heterodimers with PPARγ (Fig. 4B, lanes 1 and 2). It also had no effect on the binding of PV to PPRE (Fig. 4B, lanes 5 and 6). In contrast to T3, troglitazone (Fig. 4B, lane 4) could not release NCoR from the PPRE-bound unliganded TRβ1 (Fig. 4B, compare lane 4 with lane 3) and the PPRE-bound PV (Fig. 4B, compare lane 8 with lane 7). These findings indicate that the release of PPRE-bound TRβ1 or PV heterodimers with PPARγ is troglitazone-independent. These results suggest a polarity in the sensitivity of the ligand in releasing NCoR from the PPRE-bound unliganded TRβ1/PPARγ and PV/PPARγ and that the unliganded TRβ1 and PV play a dominant role in affecting their dimeric partner PPARγ.
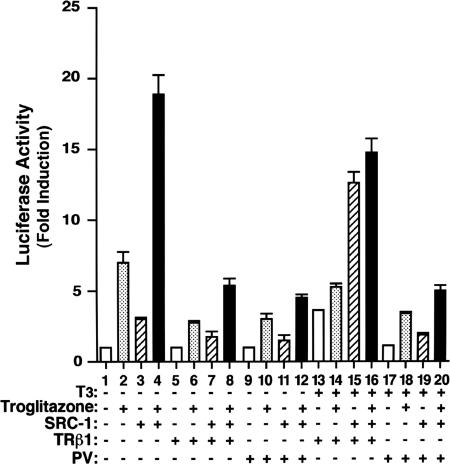
SRC1-Mediated Transactivation Activity of PPARγ Is Abrogated in the Presence of PV. SRC-1 is a coactivator for TR and PPARγ that plays a crucial role in their transcription activation upon ligand binding (2). Transcriptional activation by liganded TR is mediated through interaction with the coactivators and recruitment of histone acetyltransferase activities. The above EMSA findings predict that PPRE-bound unliganded-TRβ1/PPARγ and PV/PPARγ would fail to recruit coactivators such as the SRC-1, a coactivator for TR and PPARγ. To test this hypothesis, we carried out a transient transfection reporter assay. Fig. 5, bar 4 shows that SRC-1 enhanced the troglitazone-dependent PPARγ transactivation (2.7-fold; Fig. 5, compare bar 4 with bar 2). In the absence of T3, the unliganded TRβ1 repressed (60-70%) troglitazone-dependent PPARγ transactivation in the absence of SRC-1 (Fig. 5, compare bar 6 with bar 2) or in its presence (Fig. 5, compare bar 8 with bar 4). A similar extent of repression of the troglitazone-dependent PPARγ transactivation was observed for PV (Fig. 5, compare bar 10 with bar 2 and bar 12 with bar 4). However, in the presence of T3 and TRβ1, SRC-1 potentiated the troglitazone-independent (Fig. 5, compare bar 15 with bar 13) and -dependent (Fig. 5, compare bar 16 with bar 14) PPARγ transactivation ≈3-fold. In contrast, the SRC-1 potentiation of PPARγ transactivation was abrogated by PV, despite the presence of T3 (Fig. 5, compare bar 19 with bar 17) or together with troglitazone (Fig. 5, compare bar 20 with bar 18). Taken together, these results suggest that constitutive association of PV with corepressors prevents the recruitment of SRC-1 to the PPARγ/PV complexes in the presence of troglitazone.
Fig. 5.
PV represses the SRC1-enhanced transactivation of PPARγ. CV-1 cells were cotransfected with 0.4 μg of the reporter plasmid (pPPRE-TK-Luc), 0.2 μg of pSG5/stop-mPPARγ1 for PPARγ1, and various cDNA expression vectors [empty vector, pCLC 51 for TRβ1(1 μg), pCLC51PV for PV (1 μg), or pIRES-SRC-1 for SRC-1 (1 μg)], as indicated. Cells were treated with either DMSO as vehicle or troglitazone (20 μM) in the absence or presence of T3 (100 nM), as marked. Data were normalized against the protein concentration in the lysates. Relative luciferase activity was calculated as shown in Fig. 3. The data are expressed as mean ± SD (n = 3).
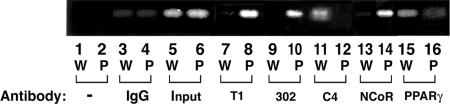
Recruitment of PV to the Promoter of a PPARγ-Target Gene, the LpL, in the Thyroid of TRβPV/PV Mice. To further support the notion that constitutive association of PV with NCoR leads to the repression of PPARγ transcription activity, we carried out the CHIP assays using thyroid nuclear extracts of TRβPV/PV mice to determine whether PV and NCoR were recruited to the promoter of the LpL gene (LpL) in vivo. LpL is a direct target gene of PPARγ that contains a PPRE in its promoter between -169 and -157 (25). Its mRNA expression is repressed in the thyroid of TRβPV/PV mice (9). Fig. 6 shows the recruitment of PV, NCoR, and PPARγ to the LpL promoter by CHIP assays. Mutant PV was clearly recruited to the LpL promoter as an 81-bp PCR product, detected when DNA-protein complexes were immunoprecipitated by either polyclonal anti-PV-specific antibodies T1 (Fig. 6, lane 8) or the monoclonal antibody #302 (Fig. 6, lane 10). As expected, no positive signals were detected in wild-type mice (Fig. 6, lanes 7 and 9). A weak signal for wild-type TRs was detected in wild-type mice (Fig. 6, lane 11) when anti-TR antibody (C4) was used in the assay but not in TRβPV/PV mice (Fig. 6, lane 12). That NCoR was also recruited to the LpL promoter was demonstrated in TRβPV/PV mice by the positive signal shown in Fig. 6, lane 14 but not in wild-type mice (Fig. 6, lane 13). A stronger signal was shown for wild-type mice (Fig. 6, lane 15) than for TRβPV/PV mice (Fig. 6, lane 16), when anti-PPARγ antibody was used to immunoprecipitate the DNA-protein complexes, indicating that less PPARγ was recruited to the LpL promoter in TRβPV/PV mice. The weaker signal detected in TRβPV/PV mice (Fig. 6, lane 16) most likely reflects the competition of PV with PPARγ for binding to LpL PPRE (see EMSA results and transcription assays in Figs. 2 and 3, respectively). Fig. 6, lanes 3 and 4 were the negative controls in which the IgGs were used for immunoprecipitation.
Fig. 6.
Recruitment of NCoR to PPRE-bound PPARγ and PV in the promoter of the LpL gene in the thyroid of TRβPV/PV mice by CHIP assay. Thyroid nuclear extracts from wild-type (W) or TRβPV/PV mice (P) were processed for CHIP assay, as described in Materials and Methods. Anti-PPARγ, anti-NCoR, anti-PV (polyclonal antibody, T1, or monoclonal antibody #302) (17) antibodies, and IgG (for negative controls) were used for immunoprecipitation. The precipitated DNA was amplified by PCR with primers specific for the LpL PPRE, and the products were analyzed. A representative example is shown here, but the experiments were repeated three times with similar results.
Discussion
This study shows complex regulation of the PPARγ activity by the liganded, unliganded, and mutated TRβ1. Consistent with findings by others (13-15, 26), the liganded TRβ1 can bind to PPRE as heterodimers with RXR. Interestingly, this study also shows that TRβ1 can bind to PPRE as heterodimers with PPARγ, even though the binding of TRβ1/PPARγ and TRβ1/RXR heterodimers is relatively weaker than the binding of PPARγ/RXR to PPRE. Consistent with the in vitro EMSA study, the cell-based study shows that the liganded TRβ1 itself can activate PPRE-mediated transcriptional activity in the absence of PPARγ ligand (troglitazone). However, in the presence of troglitazone, the T3-bound TRβ1 could inhibit troglitzone-dependent PPARγ transcriptional activity by competition with PPARγ for binding to PPRE and by sequestering RXR. This study further demonstrates the repression of the transcriptional activity of PPARγ by the T3-bound TRβ1 and PV in monkey CV-1 cells, suggesting that the crosstalk between the TR- and PPARγ-signaling pathways is not limited to thyroid cells (19). The in vitro DNA-binding study shows that the unliganded TRβ1 and PV competes with PPARγ for binding to PPRE and inhibits the binding of the transcriptionally active PPARγ/RXR to PPRE. The cell-based transcriptional analysis further shows the repression of troglitzone-dependent PPARγ transcription activity by the unliganded TRβ1 and PV. That no such transcription repression by a DNA-binding-deficient TRβ1 mutant further (data not shown) supports the critical role of competition of TRβ1 or PV with PPARγ for binding to PPRE in mediating the repression of PPARγ transcription. Further analysis indicates that the transcriptional repression is due to association of the unliganded TRβ1 or PV with corepressors, thereby resulting in failure to recruit coactivators for transcriptional activation. Thus, this study has provided insights into the molecular mechanisms by which the unliganded TRβ1 or PV negatively regulates the activity of PPARγ.
A recent report indicates that PPARγ is associated with corepressors on the promoter of glycerol kinase, a PPARγ-target gene in adipocytes (27). Binding of PPARγ ligands triggers the release of corepressors, resulting in the activation of this target gene (27). However, it is important to note that the association of a corepressor, such as NCoR, with the unliganded TRβ1 and PV results in the troglitazone-independent repression of PPARγ (Fig. 5). This effect would suggest that an association of NCoR with the unliganded TRβ1 in unliganded TRβ1/PPARγ or with PV in PV/PPARγ heterodimers prevents either the binding of troglitazone to the heterodimeric partner PPARγ or the recruitment of coactivators, such as SRC-1, to the liganded PPARγ. The former possibility is favored, because, in an analogy to RXR/TR, formation of this heterodimer is known to prevent RXR from binding its ligands (12). Although T3 can relieve the repression effect of unliganded TRβ1/PPARγ on troglitazone-dependent transcriptional activity of PPARγ by releasing corepressors, it cannot relieve the repression effect on PV/PPARγ, because PV cannot bind T3. Thus, PV is a constitutive dominant negative regulator of PPARγ action.
It has been recognized that peroxisome proliferators and T3 have overlapping metabolic effects and regulate a similar subset of genes involved in maintaining lipid homeostasis. Crosstalk between TR- and PPAR-signaling pathways has been documented by the findings that PPARα negatively regulates the expression of certain thyroid hormone target genes (13, 26), and, conversely, a PPARα-target gene, acyl-CoA oxidase, was shown to be modulated by the liganded TRα1 (15). The present findings, however, have revealed a significantly expanded scope by which TR can regulate the PPARγ-signaling pathways, in that not only the liganded TR but also the unliganded TR and mutated TR can act to affect PPARγ transcriptional activity.
That the unliganded TR and mutated TRβ repress the ligand-dependent transcriptional activity of PPARγ has important physiological implications. In rats treated with T3 to reach a hyperthyroid state, induction of a key enzyme in cholesterol metabolism, CYP4A2, by pharmacological doses of dehydroepiandrosterone (DHEA) (a peroxisome proliferating agent) is completely inhibited at the mRNA level. In thyroidectomized rats, basal expression of CYP4A2 mRNA is decreased compared with euthyroid controls (28). These in vivo observations are consistent with the regulation of PPAR-signaling pathways by the liganded and unliganded TR demonstrated in this study. In humans, overt hypothyroidism is associated with dyslipidemia. In these patients, there is an increase in serum total cholesterol, low-density lipoprotein cholesterol, apolipoprotein B, lipoprotein levels, and, possibly, triglyceride levels. These abnormalities could be due to dysfunction of T3-target genes directly mediated by TRs. However, it is entirely possible that these abnormalities are partly mediated through the repression of PPAR-target genes by the unliganded TRs. The identification of PPAR-target genes affected by unliganded TRs awaits future studies.
The in vivo functional consequence of the repression of PPARγ-signaling pathways by PV is evident in the thyroid of TRβPV/PV mice. The expression of a PPARγ downstream target gene, LpL, is down regulated 5-fold by the expression of PV in TRβPV/PV mice (9). The repression of the PPARγ-signaling pathways is one of the altered pathways that contribute to the carcinogenesis of the thyroid (9). Thus, in addition to affecting metabolic pathways and lipid homeostasis, the repression of the PPARγ-signaling pathways by PV could lead to aberrant regulation of PPARγ downstream genes to promote the development and progression of thyroid cancer.
In addition to the PV mutation, many other dominantly negative TRβ mutants are known to cause resistance to thyroid hormone (5). Their dominant negative activity is regulated by many factors, including the site of mutations and the extent of aberrant interaction with corepressors, leading to variable clinical manifestations (23, 29). These TRβ mutants, in addition to affecting the expression of T3-target genes through their dominant negative activity on the wild-type TRs, could also aberrantly affect the expression of PPARγ-target genes. Therefore, the discovery that the PPARγ-signaling pathway is negatively regulated by the unliganded and mutated TRβ broadens our understanding of the regulation of genes involved in metabolic pathways, lipid homeostasis, and carcinogenesis.
Acknowledgments
We thank Drs. Ming Tsai (Baylor College of Medicine, Houston), Frank Gonzalez (National Institutes of Health, Bethesda), L. Michalik (University of Lausanne, Lausanne, Switzerland), and Brian West (Plexxikon, Inc., Berkeley, CA) for the plasmids pIRES-SRC-1, pPPRE-TK-Luc, pSG5/stop-mPPARγ, and 3RID-CMV-FLAG-NCoR, respectively. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Author contributions: O.A., H.Y., F.F., X.Z., and S.-y.C. designed research; O.A., H.Y., F.F., and X.Z. performed research; O.A., H.Y., F.F., X.Z., and S.-y.C. analyzed data; and S.-y.C. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: CHIP, chromatin immunoprecipitation; LpL, lipoprotein lipase; NCoR, nuclear receptor corepressor; PPAR, peroxisome proliferator-activated receptor; PPRE, peroxisome proliferator response element; RTH, resistance to thyroid hormone; RXR, retinoid X receptor; TR, thyroid hormone receptor; TRE, thyroid hormone response elements; T3, 3,3′,5-triiodo-l-thyronine.
References
- 1.Cheng, S.-y. (2000) Rev. Endocr. Metab. Disord. 1/2, 9-18. [DOI] [PubMed] [Google Scholar]
- 2.Smith, C. L. & O'Malley, B. W. (2004) Endocr. Rev. 25, 45-71. [DOI] [PubMed] [Google Scholar]
- 3.Kaneshige, M., Kaneshige, K., Zhu, X. G., Dace, A., Garrett, L., Carter, T. A., Kazlauskaite, R., Pankratz, D. G., Wynshaw-Boris, A., Weintraub, B., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 13209-13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrilla, R., Mixson, A. J., McPherson, J. A., McClaskey, J. H. & Weintraub, B. D. (1991) J. Clin. Invest. 88, 2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Refetoff, S., Weiss, R. E. & Usala, S. J. (1993) Endocr. Rev. 14, 348-399. [DOI] [PubMed] [Google Scholar]
- 6.Ono, S., Schwartz, I. D., Mueller, O. T., Root, A. W., Usala, S. J. & Bercu, B. B. (1991) J. Clin. Endocrinol. Metab. 73, 990-994. [DOI] [PubMed] [Google Scholar]
- 7.Meier, C. A., Dickstein, B. M., Ashizawa, K., McClaskey, J. H., Muchmore, P., Ransom, S. C., Merke, J. B., Hao, E. U., Usala, S. J., Bercu, B. B., et al. (1992) Mol. Endocrinol. 6, 248-258. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki, H., Willingham, M. C. & Cheng, S. Y. (2002) Thyroid 12, 963-969. [DOI] [PubMed] [Google Scholar]
- 9.Ying, H., Suzuki, H., Furumoto, H., Walker, R., Meltzer, P., Willingham, M. C. & Cheng, S. Y. (2003) Carcinogenesis 24, 1467-1479. [DOI] [PubMed] [Google Scholar]
- 10.Lemberger, T., Desvergne, B. & Wahli, W. (1996) Annu. Rev. Cell Dev. Biol. 12, 335-363. [DOI] [PubMed] [Google Scholar]
- 11.Desvergne, B. & Wahli, W. (1999) Endocr. Rev. 20, 649-688. [DOI] [PubMed] [Google Scholar]
- 12.Mangelsdorf, D. J. & Evans, R. M. (1995) Cell 83, 841-850. [DOI] [PubMed] [Google Scholar]
- 13.Juge-Aubry, C. E., Gorla-Bajszczak, A., Pernin, A., Lemberger, T., Wahli, W., Burger, A. G. & Meier, C. A. (1995) J. Biol. Chem. 270, 18117-18122. [DOI] [PubMed] [Google Scholar]
- 14.Bogazzi, F., Hudson, L. D. & Nikodem, V. M. (1994) J. Biol. Chem. 269, 11683-11686. [PubMed] [Google Scholar]
- 15.Hunter, J., Kassam, A., Winrow, C. J., Rachubinski, R. A. & Capone, J. P. (1996) Mol. Cell. Endocrinol. 116, 213-221. [DOI] [PubMed] [Google Scholar]
- 16.Zhu, X.-G., McPhie, P., Lin, K.-h. & Cheng, S. Y. (1997) J. Biol. Chem. 272, 9048-9054. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, X.-y., Kaneshige, M., Kamiya, Y., Kaneshige, K. & Cheng, S.-y. (2002) Mol. Endocrinol. 16, 2077-2092. [DOI] [PubMed] [Google Scholar]
- 18.Helledie, T., Grontved, L., Jensen, S. S., Kiilerich, P., Rietveld, L., Albrektsen, T., Boysen, M. S., Nohr, J., Larsen, L. K., Fleckner, J., et al. (2002) J. Biol. Chem. 277, 26821-26830. [DOI] [PubMed] [Google Scholar]
- 19.Ying, H., Suzuki, H., Zhao, L., Willingham, M. C., Meltzer, P. & Cheng, S. Y. (2003) Cancer Res. 63, 5274-5280. [PubMed] [Google Scholar]
- 20.Bhat, M. K., McPhie, P., Ting, Y. T., Zhu, X.-G. & Cheng, S.-y. (1995) Biochemistry 34, 10591-10599. [DOI] [PubMed] [Google Scholar]
- 21.Andrews, M. T. & Brown, D. D. (1987) Cell 51, 445-453. [DOI] [PubMed] [Google Scholar]
- 22.Almouzni, G., Khochbin, S., Dimitrov, S. & Wolffe, A. P. (1994) Dev. Biol. 165, 654-669. [DOI] [PubMed] [Google Scholar]
- 23.Yoh, S. M., Chatterjee, V. K. & Privalsky, M. L. (1997) Mol. Endocrinol. 11, 470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagami, T. & Jameson, J. L. (1998) Endocrinology 139, 640-650. [DOI] [PubMed] [Google Scholar]
- 25.Schoonjans, K., Peinado-Onsurbe, J., Lefebvre, A. M., Heyman, R. A., Briggs, M., Deeb, S., Staels, B. & Auwerx, J. (1996) EMBO J. 15, 5336-5348. [PMC free article] [PubMed] [Google Scholar]
- 26.Ijpenberg, A., Jeannin, E., Wahli, W. & Desvergne, B. (1997) J. Biol. Chem. 272, 20108-20117. [DOI] [PubMed] [Google Scholar]
- 27.Guan, H. P., Ishizuka, T., Chui, P. C., Lehrke, M. & Lazar, M. A. (2005) Genes Dev. 19, 453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb, S. J., Xiao, G. H., Geoghegan, T. E. & Prough, R. A. (1996) Mol. Pharmacol. 49, 276-287. [PubMed] [Google Scholar]
- 29.Cheng, S.-y. (2005) Trends Endocrinol. Metab. 16, 176-182. [DOI] [PubMed] [Google Scholar]