Abstract
1. Intracellular recordings were made from the outer segments of rods in the isolated, superfused retina of Bufo marinus. Cells were impaled under observation with a compound microscope fitted with an infra-red image converter. Changes of membrane voltage and some concomitant changes of input resistance were measured in response to light, membrane polarization and iontophoretic injections.
2. By means of a double barrel micropipette, charge was passed into a rod from a micropipette barrel that contained Ca2+ while no net current crossed the plasma membrane. In about half the cells, immediately after the injection, a hyperpolarization was observed that decayed with a time course similar to the decay of the receptor potential.
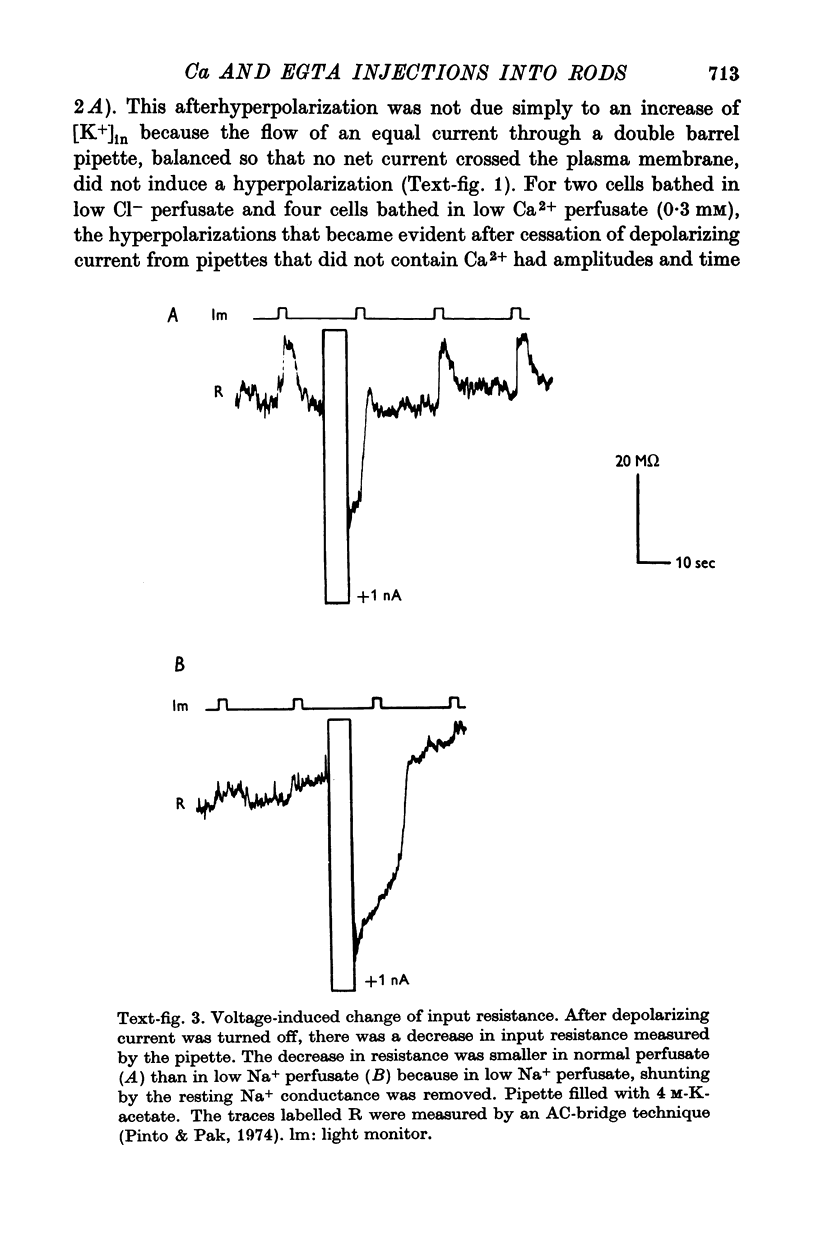
3. Membrane hyperpolarization also occurred after a depolarizing current stopped flowing into a rod through a single barrel pipette that contained only K-acetate. This hyperpolarization was accompanied by an increase of membrane conductance. The reversal potential for the conductance-increase was between the voltage in the dark and the voltage in the absence of [Na+]out. A larger hyperpolarization became evident after an equal depolarizing current stopped flowing into a rod through a pipette that also contained Ca2+; this larger after-hyperpolarization was due to both the cessation of depolarizing current and the injection of Ca2+.
4. A depolarization of 10-20 mV that lasted 2-60 sec became evident after hyperpolarizing current stopped flowing into a rod through a single-barrel pipette filled with K-EGTA. During the after-depolarization, the responses to small, dim spots of light were attenuated. No depolarization was observed after passing hyperpolarizing currents into rods through pipettes that contained either acetate-, SO2-4 or MOPS-.
5. These results show that sequestration of [Ca2+]in depolarizes the plasma membrane and that an increase in [Ca2+]in hyperpolarizes the membrane mimicking the later part of the receptor potential. These findings support the hypothesis of Yoshikami & Hagins (1971) that Ca2+ is an intracellular messenger for excitation in vertebrate rods.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. I. New evidence supporting the linkage to extracellular space of outer segment saccules of frog cones but not rods. J Cell Biol. 1968 May;37(2):424–444. doi: 10.1083/jcb.37.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Gold G. H., Dowling J. E. Receptor coupling in the toad retina. Cold Spring Harb Symp Quant Biol. 1976;40:547–561. doi: 10.1101/sqb.1976.040.01.051. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Ionic mechanisms in excitation of photoreceptors. Ann N Y Acad Sci. 1975 Dec 30;264:314–325. doi: 10.1111/j.1749-6632.1975.tb31492.x. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Proceedings: A role for Ca2+ in excitation of retinal rods and cones. Exp Eye Res. 1974 Mar;18(3):299–305. doi: 10.1016/0014-4835(74)90157-2. [DOI] [PubMed] [Google Scholar]
- Korenbrot J. I., Cone R. A. Dark ionic flux and the effects of light in isolated rod outer segments. J Gen Physiol. 1972 Jul;60(1):20–45. doi: 10.1085/jgp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A. Light-dependent Ca++ content of rod outer segment disc membranes. Invest Ophthalmol. 1974 Sep;13(9):700–701. [PubMed] [Google Scholar]
- Mason W. T., Fager R. S., Abrahamson E. W. Ion fluxes in disk membranes of retinal rod outer segments. Nature. 1974 Feb 22;247(5442):562–563. doi: 10.1038/247562a0. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969 Jul 12;223(5202):201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Pinto L. H., Pak W. L. Light-induced changes in photoreceptor membrane resistance and potential in Gecko retinas. I. Preparations treated to reduce lateral interactions. J Gen Physiol. 1974 Jul;64(1):26–48. doi: 10.1085/jgp.64.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical activity of vertebrate photoreceptors. Q Rev Biophys. 1970 May;3(2):179–222. doi: 10.1017/s0033583500004571. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Weller M., Virmaux N., Mandel P. Role of light and rhodopsin phosphorylation in control of permeability of retinal rod outer segment disks to Ca2plus. Nature. 1975 Jul 3;256(5512):68–70. doi: 10.1038/256068a0. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]