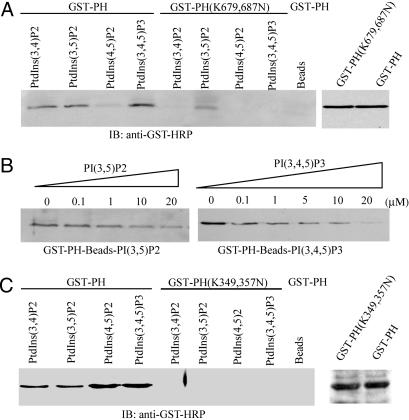
Fig. 1.
PH domain of PIKE possesses distinct phosphoinositol lipids-binding activities. (A) PIKE-L PH domain WT, but not mutant, binds to 3′-phosphate phosphoinositol lipids. Purified GST-PH WT and (K679,687N) mutant recombinant proteins were incubated with agarose-conjugated phosphoinositol lipid beads. After washing, the associated proteins of the beads were analyzed by immunoblotting with anti-GST-horseradish peroxidase Ab. WT PH domain strongly binds 3′-phosphate phosphoinositol lipids, but not PI(4,5)P2, and (K679,687N) mutation completely eliminates the binding. However, the mutant still weakly associates with PI(3,5)P2 (Left). (B) PI(3,4,5)P3 specifically binds to PH domain. GST-PH fusion proteins were coupled to PI(3,4,5)P3-conjugated beads. Different [PI(3,4,5)P3] were incubated with the complex at 4°C for 3 h. PI(3,4,5)P3 (20 μM) completely abolishes PH domain binds to PI(3,4,5)P3-conjugated beads (Right). However, the same concentration of PI(3,5)P2 fails (Left). (C) PIKE-A PH domain W,T but not (K349,357N) mutant, binds to phosphoinositol lipids. As a control, agarose beads do not bind to GST-PH fusion protein (Left). The expression of WT and mutant GST-PH domain from PIKE-A is confirmed (Right). The above experiments were repeated three times.