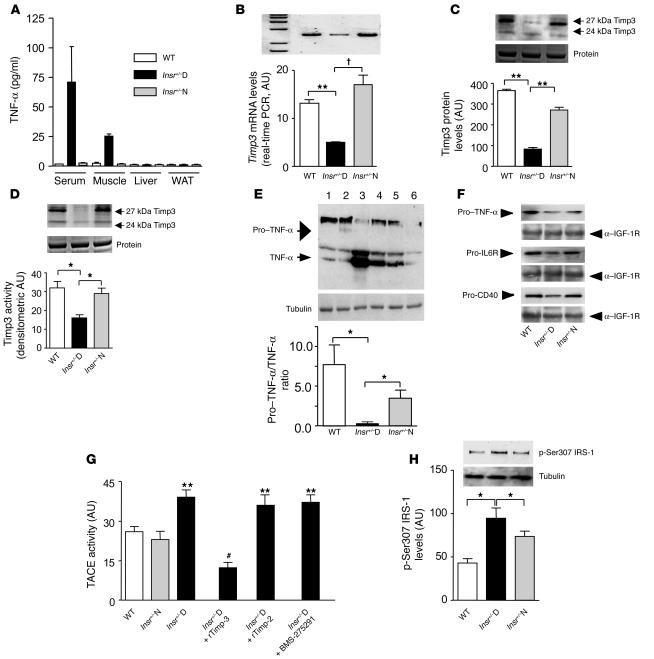
Figure 1.
Timp3 expression and activity in skeletal muscle of Insr+/–mice. (A) Quantification of TNF-α in Insr+/–D, Insr+/–N, and WT littermates. (B) Real-time RT-PCR analysis of Timp3 mRNA expression in Insr+/–D, Insr+/–N, and WT mice. **P < 0.01; †P < 0.001 by 1-way ANOVA. (C) Western blot analysis of Timp3 protein expression in Insr+/–D, Insr+/–N, and WT mice (equal loading was confirmed by silver staining). **P < 0.01 by 1-way ANOVA. (D) Comparison of Timp3 activity, measured by reverse zymography in Insr+/–D, Insr+/–N, and WT mice (equal loading was confirmed by silver staining). *P < 0.05 by 1-way ANOVA. (E) Pro–TNF-α to TNF-α conversion in Insr+/–D (lanes 3 and 4), Insr+/–N (lanes 5 and 6), and WT (lanes 1 and 2) mice. *P < 0.05 by 1-way ANOVA. (F) Shedding of ProTNF-α, pro–IL-6R, and pro-CD40 in Insr+/–D, Insr+/–N, and WT mice. Gel loading was normalized by anti–IGF-1R. (G) TACE activity measured by a fluorimetric assay in Insr+/–D, Insr+/–N, and WT mice in the presence or absence of recombinant Timp3 (rTimp-3) (100 mM), recombinant Timp2 (rTimp-2) (100 mM), and specific MMP inhibitor BMS-275291 (1 μM). **P < 0.01 versus WT; #P < 0.01 versus Insr+/–D by 1-way ANOVA. (H) IRS-1 phosphorylation on Ser307 in Insr+/–D, Insr+/–N, and WT. *P < 0.05 by 1-way ANOVA. Data from 3 separate experiments are summarized in the bar graphs and expressed as mean ± SD.