Figure 2.
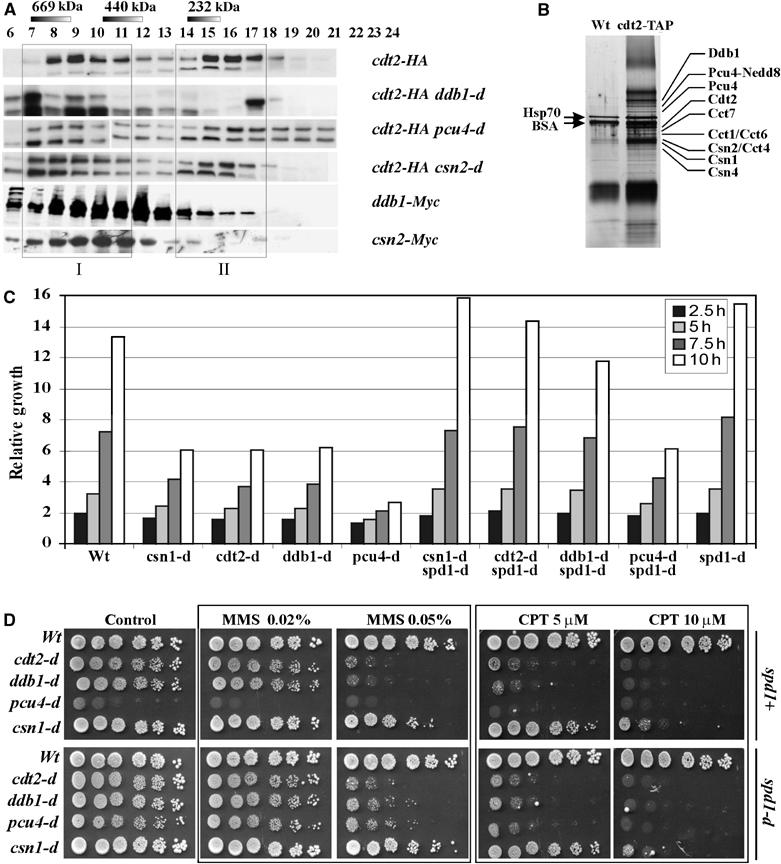
Cdt2 exists in two different protein complexes. (A) Gel filtration shows Cdt2-HA coeluting at 500–800 kDa (fraction I: FI) and 200 kDa (fraction II: FII). FI coelutes with Ddb1-Myc and Csn2-Myc. Deletion of the ddb1, pcu4 or csn2 did not result in complete Cdt2-FI loss. ddb1 deletion resulted in complete loss of Cdt2-FII. pcu4 deletion resulted in partial Cdt2-FII loss. csn2 deletion did not affect FII. (B) Silver-stained SDS–PAGE gradient gel following Cdt2-TAP purification. The four copurifying subunits (56.6–60.6 kDa) of the T chaperonin complex are indicated. The band above Ddb1 could not be unambiguously identified. Bands below Csn4 were assumed to represent other CSN subunits identified by Liu et al (2003). (C) Relative growth (cell number doublings) of strains indicated. (D) Serial dilutions of log-phase csn1, cdt2, ddb1 and pcu4 null mutant cells with (bottom) or without (top) concomitant spd1 deletion were spotted onto rich media containing indicated dosages of MMS or CPT and incubated for 4 days.
