Figure 5.
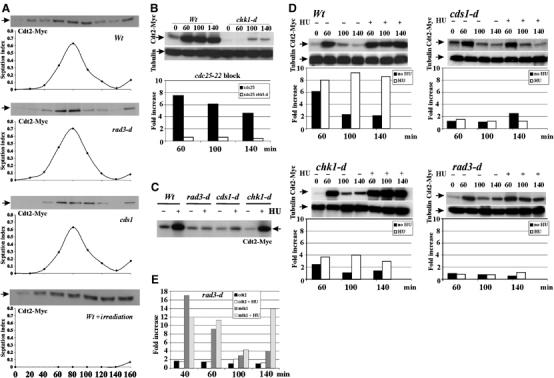
Expression of Cdt2 in normal cell cycle and upon checkpoint activation. (A) Wild-type, rad3-d or cds1-d cells (harbouring cdt2-Myc) were G2 synchronised by elutriation. Time 0: cells in the second G2 phase. First three panels: 20 min samples were taken through subsequent mitosis and S phase for extract preparation and septation index. Bottom panel: wild-type cells were irradiated (500 Gy γ-irradiation) at time 0. A 50 μg portion of total protein was separated by SDS–PAGE and probed with α-Myc. Cdt2 peaks in S phase. (B) Cdt2-Myc and cdt2 mRNA levels were analysed following γ-irradiation. cdt2-Myc cdc25-22 cells either with or without chk1 deletion were synchronised in G2 (35°C, 4 h) and irradiated (500 Gy). Total Cdt2-Myc protein and mRNA levels (relative to G2 unirradiated cells, assayed by QPCR) are shown for indicated times. chk1-d cells were defective for Cdt2 protein and mRNA accumulation. (C–E) Cdt2 protein and mRNA levels after replication stress were measured by Western blotting (α-Myc) and QPCR. α-Tubulin is used as a loading control. (C) Cdt2-Myc in unsynchronised rad3-d, cds1-d and chk1-d cells treated or not with 10 mM HU for 4 h. (D) Cdt2-Myc and cdt2 mRNA levels in synchronised wild-type, chk1-d, cds1-d and rad3-d cells. Half the sample was treated with 10 mM HU (+) during the first cell cycle to activate replication checkpoint. (E) mik1 and cdt2 mRNA in synchronised rad3-d cells either with (+) or without HU treatment.
