Figure 6.
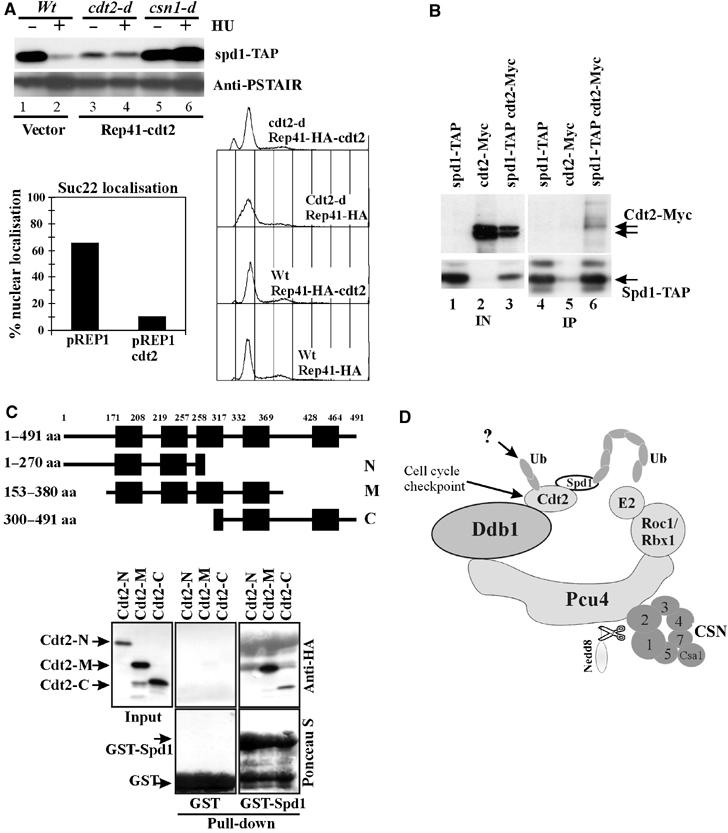
Cdt2 is an adaptor protein for Spd1. (A) Top: Rep41(HA-Cdt2) plasmid or vector control was induced by the absence of thiamine (22 h) in spd1-TAP strains shown. Half of each culture was treated (20 mM HU for 4 h). Spd1 levels were monitored by SDS–PAGE and IgG binding to TAP. α-PSTAIR (Cdc2 peptide antibody) was used as a loading control. Overexpressed Cdt2-HA (α-HA, top) caused Spd1 degradation. Overproduction of Cdt2 did not rescue the csn1-d defect for Spd1 degradation (lanes 5 and 6). Bottom left: nuclear signal of Suc22R2 was quantified in the presence of overexpressed Cdt2 (Rep1 plasmid, 22 h without thiamine). Bottom right: Cdt2-HA overexpression rescues the slow S-phase phenotype of cdt2-d cells but does not affect cell cycle profiles of wild-type cells. (B) Co-precipitation from extracts of indicated strains of Cdt2-Myc with Spd1-TAP. Strains contained the csn1-d mutation to prevent Spd1-TAP degradation. We estimated <5% of Cdt2-Myc co-precipitates. (C) Truncated HA-tagged Cdt2 constructs containing different WD40 repeat domains expressed in S. pombe from Rep41-HA. Cdt2 constructs were pulled down from soluble extracts using purified Spd1-GST (∼38 kDa) or GST alone (25 kDa). Only Cdt2-M and Cdt2-C fragments were efficiently pulled down by Spd1-GST. (D) A model of Pcu4–CSN complex containing Cdt2. Cdt2 protein is regulated by a combination of constant UPS-dependent degradation, cell cycle and checkpoint-driven transcription and an uncharacterised cell cycle-dependent post-transcriptional mechanism. Together with Ddb1, Cdt2 adapts Spd1 to the Pcu4-scaffolded RING-E2 enzyme for ubiquitylation. CSN is obligatory for Pcu4–Ddb1–Cdt2 function, possibly via regulation of neddylation and E2 loading.
