Abstract
Propranolol was used to investigate the role of phosphatidic acid (PA) and diacylglycerol in the dimorphic transition in Candida albicans. Propranolol was able to inhibit the appearance of germ tubes without decreasing growth rate. Data suggest that inhibition of morphogenesis may be due to binding by propranolol of PA derived from PLD1 hydrolysis of phosphatidylcholine.
Phospholipase D (PLD) catalyzes the hydrolysis of phosphatidylcholine to phosphatidic acid (PA) and choline (5). PA has been implicated in the regulation of differentiation pathways (1, 4) and actin polymerization (8) and can be a source of diacylglycerol (DAG) (12, 19). PLD1 activity has been demonstrated in Candida albicans (11), a pleomorphic opportunistic pathogen of humans that exhibits an ellipsoidal yeast form, an elongated pseudohyphal form, and a true hyphal form; a germ tube is a yeast-form cell that has begun to give rise to a hypha (13). PLD1 is up-regulated during the switch to filamentous growth (11). Previous studies had indicated that the addition of primary alcohol to the culture medium attenuated the appearance of germ tubes, presumably by delaying the accumulation of PLD1-derived PA due to the production of phosphatidylalcohol (11). In order to distinguish whether PA or DAG derived from PA was required for filamentation, the effect of propranolol was examined.
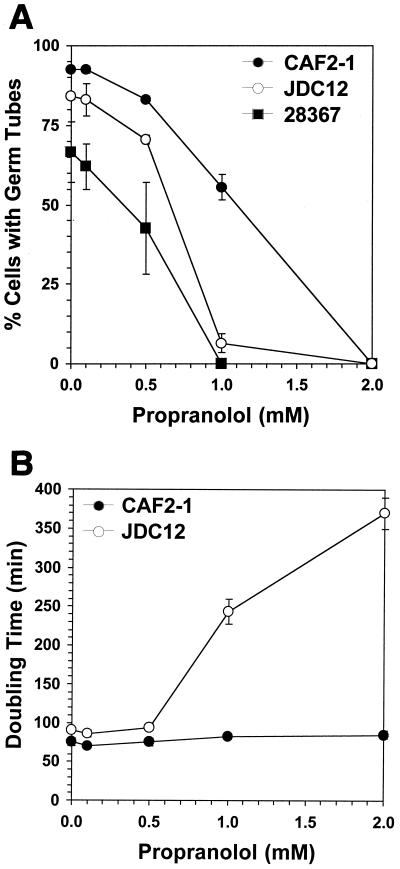
Propranolol inhibits formation of germ tubes.
The effect of propranolol on filamentous growth was measured with two wild-type strains of C. albicans, ATCC 28367 (wild-type clinical isolate [11]) and CAF2-1 (ura3::hisG/+ derivative of wild-type clinical isolate SC5314 [7]). For the growth of hyphal-form cells, a dense overnight culture was diluted to 106 cells ml−1 in yeast extract-peptone-dextrose and incubated with shaking at 38°C to induce the formation of germ tubes. Cultures treated with propranolol exhibited a dose-dependent inhibition of germ tube formation following a 2-h incubation at 38°C (Fig. 1A). Similar results were obtained when cultures were induced by both temperature shift and 10% (vol/vol) fetal bovine serum (data not shown). One explanation for the lack of germ tubes was propranolol inhibition of cell proliferation, which would have resulted in a decreased growth rate in treated cultures. The doubling time of cultures of CAF2-1 in the presence of increasing concentrations of propranolol, determined by measuring the increase in optical density at 600 nm (OD600), was unaffected by concentrations sufficient to inhibit morphogenesis (Fig. 1B). For the determination of growth rates, cultures were incubated at 30°C so that all cells would be yeast form, regardless of the presence of propranolol.
FIG. 1.
Effect of propranolol on morphogenesis and growth. (A) The percentage of cells with germ tubes in each sample was determined by direct microscopic enumeration. A minimum of 200 cells were counted for each sample. (B) Doubling times were determined by measuring the increase in OD600 of cultures incubated at 30°C. Microscopic examination of the cultures revealed the absence of germ tubes or hyphae and of clumps of cells, all factors that can invalidate the use of optical density to monitor culture growth. Each experiment was repeated at least three times, and the error bars indicate the standard errors of the means.
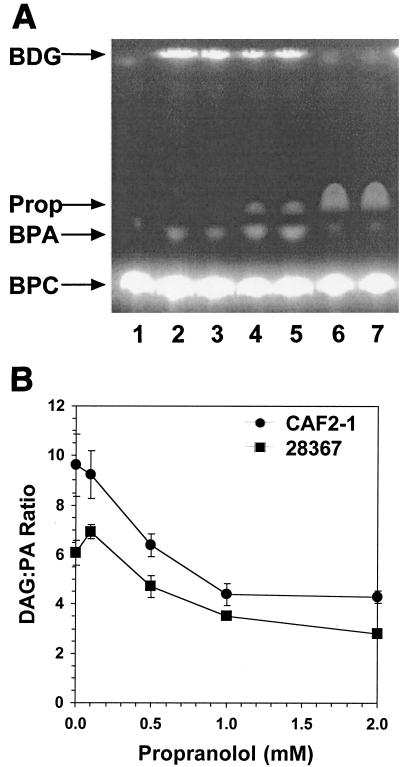
Propranolol inhibits formation of DAG from PA.
In mammalian cells, low concentrations of propranolol (0.05 to 0.2 mM) have been used to inhibit the lipid phosphate phosphohydrolases (LPPs) that convert PA into DAG (2, 10). In Saccharomyces cerevisiae, the concentration necessary to inhibit LPP is significantly higher (4). To determine whether propranolol inhibits C. albicans LPP, PLD1 assays were performed in the presence of propranolol (Fig. 2A). Extracts were prepared and PLD1 activity was measured with the fluorescent substrate BODIPY-phosphatidylcholine, which was hydrolyzed to BODIPY-phosphatidic acid (BPA) (4). Dephosphorylation of BPA by LPP yielded BODIPY-diglyceride (BDG). Products were separated by thin-layer chromatography (TLC) (6) and quantitated with a Molecular Dynamics FluorImager. PLD1 activity was expressed in arbitrary units as follows: for each TLC plate, fluorescence of the BPA spot in the control sample was set equal to 1.0 and fluorescence of the BPA spots in experimental samples was normalized to that value. Analysis of JDC12 (pld1Δ homozygote) revealed that all of the BDG detected by this in vitro assay was derived from BPA (9). The fluorescence measured in the BDG spot was divided by that of the corresponding BPA spot from the same sample to yield the BDG/BPA ratio at each propranolol concentration. As the concentration of propranolol increased, this ratio decreased, consistent with inhibition of LPP (Fig. 2B). The C. albicans LPP required high concentrations of propranolol (1.0 to 5.0 mM) for inhibition. At the highest concentrations of propranolol, the ratio did not change due to inhibition of PLD1 activity, leading to a decrease in both BPA and BDG (Fig. 2A, lanes 6 and 7).
FIG. 2.
Propranolol altered the ratio of DAG to PA. (A) The presence of propranolol in in vitro PLD1 assays increased the yield of BPA at the expense of BDG. Lane 1, no extract; lanes 2 and 3, extract without propranolol; lanes 4 and 5, extract with 1 mM propranolol; lanes 6 and 7, extract with 5 mM propranolol. Propranolol (Prop) exhibited intrinsic fluorescence when the TLC plate was scanned to detect the BODIPY-labeled lipids. (B) The ratio of DAG to PA decreased as the concentration of propranolol increased. Each experiment was repeated at least three times, and the error bars indicate the standard errors of the means.
Cells lacking PLD1 activity appear more sensitive to propranolol.
Inhibition of morphogenesis by propranolol and by primary alcohols suggests that DAG derived from PLD1-produced PA is the critical product of PLD1 with regard to this process. The loss of PLD1 activity was expected to render cells more sensitive to propranolol due to the decrease in cellular PA levels, resulting in less substrate available for the production of DAG and a more profound effect caused by propranolol. Consistent with this hypothesis, propranolol was found to inhibit completely the formation of germ tubes in strain JDC12 with a 50% inhibitory concentration (IC50) of 0.7 mM, while the IC50 for CAF2-1 was 1.15 mM (Fig. 1A). As with the wild type, the PLD1-deficient strain could fail to form germ tubes because of an inhibition of cell proliferation. In contrast to the wild-type strain, growth of the PLD1-deficient strain was dramatically impaired by propranolol (Fig. 1B). The generation time of the mutant in the presence of 2 mM propranolol was approximately four times as long as that for the untreated control. Thus, loss of PLD1 activity appears to render cell growth more sensitive to propranolol. Nevertheless, at 1 mM propranolol, cells remain viable and still fail to form germ tubes. These data are consistent with earlier studies that demonstrated a role for PLD1 in morphogenesis.
Overall DAG levels are unaffected.
Inhibition of a cellular process by propranolol is frequently taken to mean that the essential molecule is DAG rather than PA. An alternative mechanism by which propranolol could block the formation of DAG is direct binding of the drug to PA, rendering the PA inaccessible to LPP or other downstream targets. To distinguish between these two mechanisms, the concentration of DAG was measured in cells that had been treated with propranolol. Lipids were extracted by the method of Bligh and Dyer (3) following growth for 2 h in the presence or absence of 2 mM propranolol. DAG levels were quantitated with a BioTrak DAG assay kit (Amersham Pharmacia). The presence of propranolol did not alter the total amount of DAG in cells: the relative amount of DAG in the treated cells was 92% of that present in untreated cells. The lack of a significant effect on in vivo DAG levels suggested an alternative mechanism for propranolol.
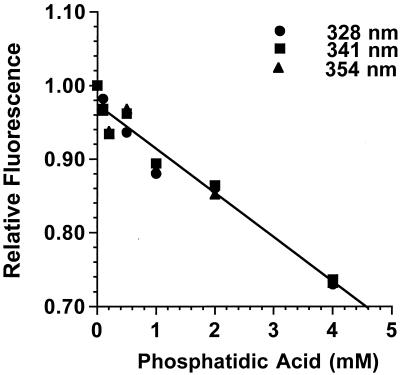
Propranolol binds to PA.
Fluorescence spectroscopy of propranolol revealed the drug bound directly to a number of phospholipids (16). To measure the fluorescence in the presence of PA, stocks of propranolol (10 mM) and PA (5 mM; dipalmitoylphosphatidic acid; Sigma-Aldrich) were prepared in 50 mM Tris-Cl (pH 7.2)-100 mM NaCl. Samples of 0.5 mM propranolol and 0 to 4 mM PA in 150 μl were analyzed with an SLM Instruments 8100 spectrofluorimeter, using an excitation wavelength of 290 nm. Figure 3 shows that the fluorescence of propranolol was quenched by PA in a concentration-dependent manner. The quenching argued that propranolol was inhibiting morphogenesis by binding to, and thus sequestering, PA. A likely source of this PA was hydrolysis of phosphatidylcholine by PLD1, in light of the increased sensitivity to propranolol of the pld1Δ mutant. Furthermore, the cellular DAG levels argue that it was the loss of PA, not an insufficiency of DAG, that was responsible for the effect of propranolol.
FIG. 3.
PA quenches the intrinsic fluorescence of propranolol in a concentration-dependent manner. The emission spectrum of propranolol has maxima at 328, 341, and 354 nm in response to excitation at 290 nm. The peak fluorescence of 0.5 mM propranolol was measured at increasing concentrations of PA and normalized to the fluorescence measured in the absence of the drug.
Propranolol has been used extensively to investigate the relative importance of PA and PA-derived DAG in several systems (2, 4, 10). The pharmacology of propranolol illustrates an important problem associated with drugs that can interact with lipids. At low concentrations, propranolol inhibits mammalian LPPs. At higher concentrations, propranolol acts as a β-adrenergic receptor antagonist. At still higher concentrations, this drug produces localized anesthetic effects, possibly due to its ability to alter membrane structure leading to depolarization (15, 18). Propranolol has an affinity for acidic phospholipids, binding to phosphatidylserine and PA (16). A diet rich in fats (14) and disease states such as obesity and hyperlipidemia (17) can alter the bioavailability and efficacy of lipophilic drugs. The dose-dependent responses to propranolol, coupled with its binding to and sequestration in lipids, can complicate the clinical administration of such a drug and confuse the interpretation of experimental results.
The mechanism by which propranolol is acting to affect morphogenesis and cell proliferation is currently under continued investigation. Regardless of the exact nature of this inhibition, the combined results of this study and earlier work (6, 11) reveal the potential significance of lipid-mediated signal transduction and metabolism in the expression of virulence traits by C. albicans. Therefore, agents like propranolol that interfere with the actions of phospholipids possess potential antifungal activity and, thus, identify many possible targets for new therapeutic agents.
Acknowledgments
This work was supported in part by the Medical University of South Carolina Institutional Research Funds of 1996-97 and by grant no. 97-151-0-IRG from the American Cancer Society.
REFERENCES
- 1.Amsterdam, A., A. Dantes, and M. Liscovitch. 1994. Role of phospholipase-D and phosphatidic acid in mediating gonadotropin-releasing hormone-induced inhibition of preantral granulosa cell differentiation. Endocrinology 135:1205-1211. [DOI] [PubMed] [Google Scholar]
- 2.Balboa, M. A., J. Balsinde, and E. A. Dennis. 1998. Involvement of phosphatidate phosphohydrolase in arachidonic acid mobilization in human amnionic WISH cells. J. Biol. Chem. 273:7684-7690. [DOI] [PubMed] [Google Scholar]
- 3.Bligh, E. C., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 4.Ella, K. M., J. W. Dolan, and K. E. Meier. 1995. Characterization of a regulated form of phospholipase D in the yeast Saccharomyces cerevisiae. Biochem. J. 307:799-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Exton, J. H. 1990. Signaling through phosphatidylcholine breakdown. J. Biol. Chem. 265:1-4. [PubMed] [Google Scholar]
- 6.Gadd, G. M., and S. A. Foster. 1997. Metabolism of inositol 1,4,5-trisphosphate in Candida albicans: significance as a precursor of inositol polyphosphates and in signal transduction during the dimorphic transition from yeast cells to germ tubes. Microbiology 143:437-448. [DOI] [PubMed] [Google Scholar]
- 7.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 8.Ha, K. S., and J. H. Exton. 1993. Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIC9 fibroblasts. J. Cell Biol. 123:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hube, B., D. Hess, C. A. Baker, M. Schaller, W. Schafer, and J. W. Dolan. 2001. The role and relevance of phospholipase D1 during growth and dimorphism of Candida albicans. Microbiology 147:861-867. [DOI] [PubMed] [Google Scholar]
- 10.Liscovitch, M., and A. Amsterdam. 1989. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. Possible role in signal transduction. J. Biol. Chem. 264:11762-11767. [PubMed] [Google Scholar]
- 11.McLain, N., and J. W. Dolan. 1997. Phospholipase D activity is required for dimorphic transition in Candida albicans. Microbiology 143:3521-3526. [DOI] [PubMed] [Google Scholar]
- 12.Morlock, K. R., J. J. McLaughlin, Y. P. Lin, and G. M. Carman. 1991. Phosphatidate phosphatase from Saccharomyces cerevisiae. Isolation of 45- and 104-kDa forms of the enzyme that are differentially regulated by inositol. J. Biol. Chem. 266:3586-3593. [PubMed] [Google Scholar]
- 13.Odds, F. C. 1988. Candida and candidiosis. Bailliere Tindall, London, England.
- 14.Ogiso, T., M. Iwaki, T. Tanino, R. Kawafuchi, and S. Hata. 1994. Effect of food on propranolol oral clearance and a possible mechanism of this food effect. Biol. Pharm. Bull. 17:112-116. [DOI] [PubMed] [Google Scholar]
- 15.Sasa, M., B. P. Avner, and E. X. Albuquerque. 1973. Actions of beta-blocking agents on membrane excitability of the lobster giant axon. Eur. J. Pharmacol. 23:97-103. [DOI] [PubMed] [Google Scholar]
- 16.Surewicz, W. K., and W. Leyko. 1981. Interaction of propranolol with model phospholipid membranes. Monolayer, spin label and fluorescent spectroscopy studies. Biochim. Biophys. Acta 643:387-397. [DOI] [PubMed] [Google Scholar]
- 17.Wojcicki, J., V. Sulzyc-Bielicka, J. Kutrzeba, B. Gawronska-Szklarz, M. Drozdzik, and Z. Sterna. 1999. Studies on the pharmacokinetics and pharmacodynamics of propranolol in hyperlipidemia. J. Clin. Pharmacol. 39:826-833. [DOI] [PubMed] [Google Scholar]
- 18.Wu, C. H., and T. Narahashi. 1973. Mechanism of action of propranolol on squid axon membranes. J. Pharmacol. Exp. Ther. 184:155-162. [PubMed] [Google Scholar]
- 19.Wu, W. I., Y. P. Lin, E. Wang, A. H. Merrill, Jr., and G. M. Carman. 1993. Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by sphingoid bases. J. Biol. Chem. 268:13830-13837. [PubMed] [Google Scholar]